Since the advent of modern medicine, our biggest strides have come from finding effective countermeasures against pathogens like bacteria and viruses. Collectively, such pathogens had been responsible for sowing history’s deadliest illnesses from the plague, to tuberculosis, smallpox, and the flu. Ever since the invention of antibiotics and vaccines, however, we’re no longer as defenseless against these pathogenic diseases as we once were. But as humans began to live longer, new diseases took their place.
The most intractable contemporary diseases are those produced by the body itself, where either the body begins to treat its own tissue as a pathogen (auto-immune diseases like Type 1 diabetes, arthritis, multiple sclerosis, and Alzheimer’s), or where cells begin to grow uncontrollably (cancer).To date, pharmaceuticals have only proven capable of either slowing the progression of these conditions or of subduing their side effects, but have limited effectiveness against their underlying causes. To change this, an entirely new approach is needed: cell therapies.
Cell therapies promise a total paradigm shift within medicine. Rather than relying on drugs for treatment, the idea behind cell therapies is that the cure-all for any disease can already be found within our existing cells. By genetically enhancing our cells, emerging research points to the fact that nearly any condition – from organ failure to auto-immune disease, and even cancer – might soon be a problem of the past. Cell therapies wouldn’t only transform the landscape of modern illness, but could completely change the medical industry as we know it.
Current Status of Cell Therapies
Just as there are millions of different cells in the human body, there are many types of cell therapies. However, the most well-studied and powerful types can be bucketed into two categories: stem cell therapies and immune cell therapies.
Stem Cell Therapies
Every human being begins as a bundle of stem cells. In the womb, personhood evolves from a small group of undifferentiated cells into a complex system of specialized skin cells, nerve cells, and muscle cells which ultimately make us up. While most cells have completed their differentiation by birth, some undifferentiated stem cells remain. These stem cells can actually be found in most of the tissues that are able to naturally repair themselves like skin, bones, or even the linings of certain organs like the liver and intestines.
Harnessing the power of these cells to repair and regenerate tissue has opened up many exciting prospects in medicine. Take, for instance, heart disease. Heart disease is the leading cause of death for most men and women in America. Though surgery and treatment can help a patient survive a heart attack, damaged heart tissue can’t be regrown. Instead, rigid scar tissue grows in its place, which eventually makes the heart stiff and incapable of properly pumping blood through the body. Apart from a full heart transplant, traditional medicine, and surgery, there are limited options for patients experiencing heart failure.
However, stem cell therapies offer a promising new avenue for regenerating tissue that can’t naturally heal on its own. A number of small-scale experimental trials have demonstrated the potential of stem cells to help regrow healthy heart tissue or limit the amount of scar tissue produced after a heart attack.
One trial in 2012 did so by extracting a patient’s own stem cells, differentiating them into heart tissue, and re-injecting them back into areas with heart damage. After six months, the patients who received this therapy showed reductions in scar mass and increases in viable heart mass. However, not all trials have yielded straightforward results. Another experimental trial concluded just one year later, showed no major improvements in patients who received bone marrow-derived stem cells injected into damaged heart tissue. Despite the conflicting results here, researchers are holding out hope — but the small scale of experimental trials makes it difficult to identify exactly what’s going on when things go right, and what’s going wrong when trials don’t produce desired outcomes.
Of course, it’s not just hearts that should respond to this type of treatment. There are incredible examples of doctors fully regrowing organs like the esophagus and even the bladder using stem cells. However, so far these have all been one-offs. These treatments are novel and dangerous and are thus only approved by the FDA for patients with few other options.
With that said, the promise of stem cell therapies is hard to ignore. for those who can afford it, domestic regulation isn’t much of an issue. Notable athletes like Peyton Manning have turned to stem cell therapy in moments of dire need. After injuring his neck multiple times and receiving numerous surgeries of no avail, Manning flew to Europe in 2011 to receive an experimental treatment that used stem cells to treat his neck injury. He hasn’t had trouble with his neck since and went on to become one of the most decorated quarterbacks in American football.
Stem cell therapy, if it can realize its potential, will have a massive role to play in the future of medicine. Stem cells are emerging as a fascinating new medical primitive, functioning as a key construction material for the human organism.
Immune Cell Therapies
The immune system is the body’s natural defense mechanism against disease. However, when our natural defenses aren’t able to eradicate disease-causing agents like cancer cells unaided, cell therapies can be used to turbocharge the performance of immune cells.
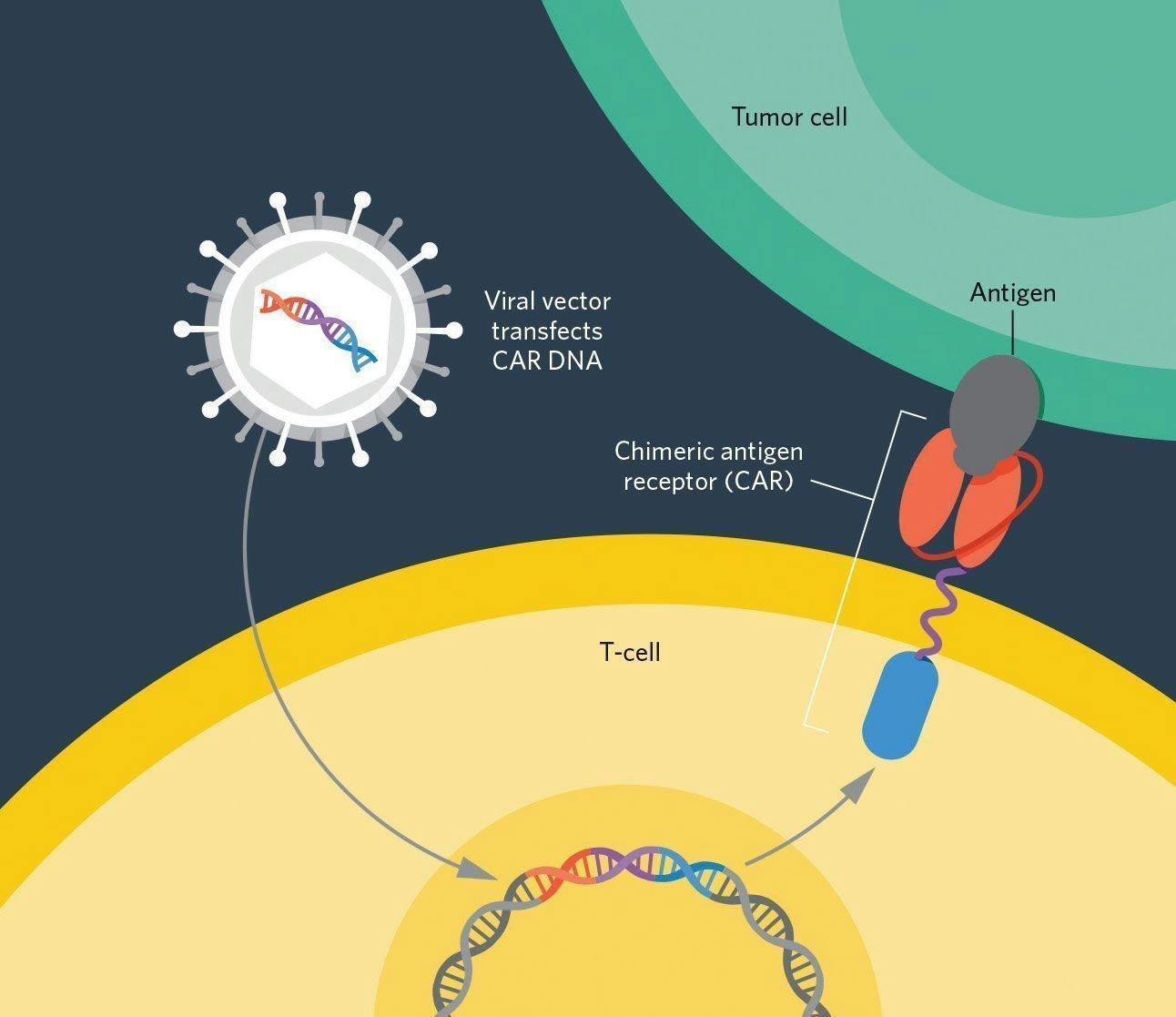
In the last decade, immune cell therapies have shown tremendous promise. The most effective immune cell therapy to date has been CAR T-cell therapy, a type of cell therapy capable of treating various blood cancers like B-cell leukemia, lymphoma, and myeloma. CAR T-cell therapy begins with the T-cells, which comprise the body’s defense mechanism against infection, cancer, and other kinds of maladies. T-cells identify and destroy harmful antigens in the body. Frustratingly, for years now, aggressive cancers have succeeded at overwhelming the human immune system and preventing T-cells from removing them. However, in the late 1980s, an Israeli scientist named Dr. Zelig Eshhar wondered if it would be possible to genetically engineer a batch of T-cells to retrofit them with a receptor designed to specifically detect cancer cells.
Dr. Esharr designed this exact receptor — the CAR or chimeric antigen receptor — in two parts. One part of the receptor called the antigen-binding domain, sticks out from the T-cell’s membrane and sets off an alarm if it detects a targeted antigen. That alarm then activates the second part of the receptor inside the T-cell, called the signaling domain, which instructs the T-cell to attack the antigen it's bound to.
Source: The Scientist
The only problem was that the technology to actually reprogram cells to express these receptors didn’t exist. Though Dr. Esharr helped write the genetic code for this new receptor, it would take decades for our abilities in genetic engineering to catch up with his ideas.
However, in 2010, the first successful demonstration of CAR T-cell therapy finally took place in Dr. Carl June’s lab at the University of Pennsylvania. The patient who underwent the trial therapy, Bill Ludwig, had been suffering from chronic lymphocytic leukemia for a decade before he was enrolled in this historic trial. June’s lab first collected Ludwig’s T-cells and genetically modified them with a CAR specifically designed to look for the markers on cancerous blood cells. Then, these modified T-cells were re-injected back into Ludwig to do their work. Remarkably, just one month after the procedure, Bill Ludwig was cancer free.
The magic of CAR-T therapies, and immune cell therapies in general, is that one dose is often enough to fully cure a patient of a disease. As modified T-cells live on in the patient’s body, they continually hunt for cancerous blood cells and destroy them upon contact. Since 2017, six CAR T therapies have been approved in the United States to treat different forms of leukemia, lymphoma, and myeloma with incredible results. Certain studies show that 90% of patients whose leukemia did not respond to traditional treatments had full remission with CAR T-cell therapy.
However, there are still significant challenges ahead. Though CAR T therapies are very good at fighting blood-based cancers, they’re not yet good at fighting solid tumors — the most common form of cancer by far. Large cancerous masses repeatedly set off T-cells antigen receptors, so much so that after a while, T-cells experience a syndrome called T-cell exhaustion, where their ability to attack the tumor declines. This is one of the reasons it is so difficult for our immune system to naturally fight off cancers. Figuring out how to bolster these therapies to destroy cancerous masses is the next milestone to unlock. Luckily, Carl June is holding out hope that we might reach that milestone in the coming decade.
Other challenges within immune cell therapies involve work on regulatory T-cells. Tregs, as they’re colloquially called, actually help suppress the body’s natural immune response. It’s an important function that ensures that the immune response doesn’t harm the body’s own tissues. All auto-immune diseases, from Type 1 diabetes to multiple sclerosis and even rheumatoid arthritis, are examples of what can happen when this Treg function breaks down. Some of the most exciting recent research in immune cell therapy involves finding ways to genetically enhance the function of Tregs such that destructive auto-immune responses can be tamed. Figuring out how to do this properly could potentially solve the puzzle of curing auto-immune disease once and for all.
Stem cell and immune cell therapies are only scratching the surface of new biotech frontiers scientists are exploring in labs across the world. Another notable approach being pursued intensely is the domain of tissue engineering.
A Brief History of Cell Engineering
The idea of using cells as therapeutic agents began in the mid-20th century. Arguably, one of the first ‘cell therapies’ has been around since the 1950s, and involves the practice of performing bone marrow transplants in patients with leukemia.
Even though these therapies worked, physicians could only theorize as to why. Outside of macroscopic procedures like bone marrow transplants, the fine structures and inner workings of our bodies eluded us. Luckily, the coming decades were very a productive time in medicine and physiology, with each decade unlocking a critical piece of the cell therapy puzzle. By the end of the 50s, James Watson and Francis Crick discovered the structure of DNA. In the 60s, Ernest McCullough and James Till at the University of Toronto proved the existence of stem cells and produced proof of cells with the ability to differentiate the function. One of the most important decades, however, was the 1970s. That’s when progress in genetic engineering first started to take off. The first genes were isolated in 1973, and by the 1980s, DNA sequencing technology gave us the ability to read and decipher any genetic code.
Though a few successful techniques of gene editing were developed in the late 80s and 90s, these techniques were limited in their precision and were labor-intensive and time-consuming to produce. The massive breakthrough for cell therapies came in 2012 when Jennifer Doudna and Emmanuelle Charpentier demonstrated that CRISPR-Cas, a mechanism used by bacteria to fight viral infections, could be re-appropriated to edit genes in any cell. The discovery which won Doudna and Charpentier a Nobel Prize showed how a specific RNA molecule could be designed to guide the CRISPR protein to a specific DNA sequence, allowing for targeted gene editing.
Since then, CRISPR has become the go-to tool for genetic editing due to its efficiency and versatility and has unlocked near-limitless potential for the field of cell therapies. If the 20th century was marked by us learning to read and write genetic code, the 21st century, largely on the back of CRISPR technology, will be marked by our ability to actually implement and run the genetic code in real cells.
The Future of Cell Therapies
From Autologous to Allogeneic
Injecting a patient with engineered cells is always risky. As already mentioned, the human immune system is very sensitive, and not always welcoming to the introduction of unfamiliar biological material. To overcome this problem, most cell therapies use the patient’s own modified cells, which is a technique known as autologous cell therapy. Using the patient’s own cells increases the chance that the patient’s organism will accept the cell injection, however, it makes the cell therapy treatment on the whole more time intensive and much more expensive since each patient requires their own unique therapy.
One method of decreasing the cost and increasing the availability of these therapies would be to find a way of producing an off-the-shelf cell therapy that could work for all patients. An off-the-shelf solution would need to solve two big problems. The first problem involves producing enough stem cells or immune cells to be used at scale. The second problem involves figuring out a mechanism such that these mass-produced, modified cells are actually accepted by the patient.
Research is still ongoing on the second question, as companies like Allogene work on strategies to decrease the immune response from allogeneic cell treatment. However, the challenge of scaling cell production might already have a promising solution.
In 2012, scientists John Gurdon and Shinya Yamanaka won a Nobel Prize for their discovery of induced pluripotent stem cells. Essentially, these scientists discovered that through chemical coaxing, common cells like skin cells or blood cells could be transmuted into a pluripotent stem cell state, which means they would have the ability to differentiate into almost any cell type in the body. This technique essentially amounts to a biological time machine that sends differentiated adult cells back into an embryonic state.
The ability to do this is particularly useful because once skin cells are converted into stem cells, those stem cells can be differentiated into any cell we want, including T-cells, Tregs, or what have you. The discovery of induced pluripotent stem cells, or iPSCs, amounts to the discovery of a mechanism that can convert adult skin or blood cells into any kind of type of cell we need to conduct cell therapies. With this technique, as long as we have abundant blood banks, we should have enough supply to produce the vehicles for cell therapy.
The discovery of iPSCs also solved an intractable ethical dilemma at the center of stem cell therapy research. For a long time, stem cells could only be acquired from the cells present in aborted fetuses. The shaky ethics of harvesting stem cells from these sources resulted in a near-decade-long cooling of stem cell research in the United States, which lasted from 2001 to 2009. During this period, the US federal government abstained from funding any stem cell-related research. Remarkably, with the discovery of iPSCs, even the Vatican has since become a vocal supporter of stem cell research, hosting multiple conferences devoted to promoting the field.
Scaling Cell Therapies
Another temporary inefficiency in the world of cell therapies is that currently because there are so few FDA-approved cell therapies, therapy designers must also operate as manufacturers for all patients across the country. This makes the manufacture of cell therapies very expensive on a per-unit basis. The situation is equivalent to the early days of the microprocessor industry when chip designers like Intel were also responsible for manufacturing their own chips. Now that the microchip industry has scaled to a sufficient size, it’s much more convenient for firms to specialize in either designing or building chips. As such, most chip design shops now outsource the manufacturing of their chips to specialized, external factories.
In a world where allogeneic or off-the-shelf cell therapy treatments become a reality, a similar shift will need to occur in the cell therapy industry. Luckily, there are already companies working on developing the biomanufacturing technology capable of supplying this need. Mammoth Biosciences and Synthego are just two of the firms working on this problem. Both are leveraging CRISPR technology to build out biological assembly lines where large numbers of cells could be genetically engineered at scale.
Increasing the Pace of Progress
Progress in innovative therapeutic treatments can only move as fast as the research progresses. However, there’s currently a big mismatch between the ways clinical trials are conducted and the types of clinical trials we’re trying to run. The FDA, for instance, was established to deal primarily with drugs and devices, and the stages of clinical trials designed to test drug or device-based interventions. Initially, a number of pre-clinical studies are conducted in the lab or on non-human subjects to assess the safety of the treatment. After the pre-clinical steps, there are four FDA-mandated clinical trial phases conducted on human patients, with each trial testing larger and larger patient populations.
However, when a study concerns the function of modified cells themselves, pre-clinical studies conducted in petri dishes or in mice don’t lend the kind of useful insights that might help these therapies succeed in later-stage clinical trial phases. To date, the most instructive research occurs in very small-scale experimental trials approved for patients in dire conditions. There are, however, proposals that envision a smarter way of experimenting with cell therapies going forward. In the near future, we could use emerging technology like 3D organ printers to supply us with realistic canvases on which scientists could more directly test the efficacy of cell therapies. Already, companies like Organovo are working on 3D printing various human tissues for use in drug testing.
The success of cell therapies at scale has the potential to transform the field of medicine, leading to a paradigm shift in how healthcare is delivered, reimbursed, and experienced by patients. While there are still challenges to overcome, including regulatory and logistical hurdles, the promise of cell therapies as a revolutionary approach to treating disease is undeniable.
Below are the companies leveraging cell therapy technology to build the future of medicine:
- Asimov is building tools to design and manufacture genetically enhanced therapeutics.
- Biostage is using a patient’s own stem cells to create organ scaffolds to help with organ replacement.
- Vericel is developing cell therapy products for sports-related injuries and severe burns.
- Mesoblast is developing allogeneic cell therapy treatments for difficult inflammatory diseases.
- Fate Therapeutics is fabricating allogeneic cell therapies to treat a range of immune disorders.
- Cellares is developing a turnkey manufacturing solution for cell therapy production.
- Deep Genomics is using AI to identify new receptors and genes to design the next generation of cell therapies.
- Mammoth Biosciences is building out a biomanufacturing platform leveraging CRISPR technology.
- Synthego is designing a CRISPR production platform capable of scaling up the production of cell therapies.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly, Fund of Fund investments and investments in which total invested capital is no more than $50,000) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.