Each year, roughly 40 billion tonnes of CO2 are emitted into the atmosphere. Given that global energy consumption increases at a rate of 2% every year, it’s likely that our emissions will increase at a similar rate.
This trajectory conflicts with the Paris Climate Agreement’s 2015 commitment to keep average global temperatures from rising more than 2 degrees Celsius — a goal that climate scientists agree will require net negative emissions. This means that merely reducing existing emissions or switching to less polluting fuel alternatives won’t be enough. We will need a solution to actively take excess carbon dioxide out of the atmosphere.
However, this problem also presents an interesting opportunity. For the past couple of decades, the world has come to look upon atmospheric carbon as a terrible pollutant. However, recent developments in the fields of carbon removal and carbon utilization are increasingly encouraging us to change our lens and instead, look upon carbon emissions as an industrial material in disguise.
If the 20th century was marked by Fritz Haber discovering how to pull nitrogen out of thin air to produce ammonia for use in fertilizer, this might be the century where we learn to pull excess carbon out of the atmosphere to make the commercial materials of the future.
Carbon Removal Techniques
There are two main approaches to removing carbon from the atmosphere. The first is called carbon capture. Although this sounds like an all-encompassing term, it technically only applies to processes that capture carbon emissions directly from power plants. To do this, power plants are retrofitted with systems that capture the exhaust gas generated by the power plant. The carbon in the exhaust gas is then compressed and transported to be processed or stored.
Though carbon capture is an important technology to reduce the amount of carbon dioxide accumulating in the atmosphere, power plants only account for 25% of total annual carbon emissions. The lion’s share of carbon emissions (roughly 60%) are produced by agriculture, transportation, and heavy industry (e.g. metallurgy plants or chemical refineries). The only way to remove carbon emitted from these sources is by directly removing carbon from the air – a strategy known as direct air capture.
Extracting carbon dioxide right out of the air was an idea first suggested by a physicist named Klaus Lackner in 1999. He realized that in the same way a sponge can absorb liquid, there exist other materials that are particularly well-suited to absorb gases — including greenhouse gases. His basic idea was to use fans to suck in air containing carbon dioxide and blow it over materials called sorbents that would attract the CO2.
Over the next few years, Lackner was one of the first to build a direct air capture prototype like this. A few companies like Climeworks, which were inspired by his ideas, soon followed suit. But given the small scale of these projects, the cost of operating them was astronomically high — $600 per ton of CO2 collected. Given that the ideal goal of direct air capture is to remove most of the excess emissions discharged annually, $600 per ton would put the annual cost of doing so at around $14 trillion.
However, direct air capture started looking a lot more promising when a Canadian company called Carbon Engineering published a paper championing a new direct air capture technique. When operating at scale, this new technique promised to decrease the cost of capture to just $94-232/ton and eventually, capture up to one million tons of CO2 per year.
Though this would be an impressive achievement, it would still only cover the cost of getting the carbon out of the air. To make paying such a price worth it, however, numerous companies have begun investigating methods of carbon utilization— the process of turning carbon collected by direct air capture into useful products like synthetic natural gas or small-chain hydrocarbons. In fact, as part of one of its pilot plants, Carbon Engineering built a mechanism that could turn captured carbon into compounds that could eventually be turned into gasoline, diesel, and even jet fuel.
Of course, removing carbon to produce more combustible fuels is only carbon-neutral, meaning it doesn’t release extra carbon into the atmosphere. To really mitigate the effects of climate change, a net-negative carbon strategy would be needed; one which would store carbon in geological or deep-ocean deposits for hundreds of years.
Carbon Engineering already has a scheme like this in the works. It’s collaborating with another company called Storegga Geotechnologies to build Europe’s largest direct-air-capture plant and store the captured carbon beneath the floor of the North Sea. The plant is expected to become operational in 2026, and the company intends to sell the sequestered carbon as carbon credits. However, concerns still abound regarding the environmental impact of placing massive carbon stores at the bottom of our largest bodies of water.
The Carbon Cycle
Carbon cycles through the environment naturally. Before there was any life on Earth, carbon rotated from under the crust of the Earth up to the surface and eventually into the atmosphere through volcanic eruptions and tectonic shifts. The carbon would then collect in the oceans, mixing with water to form carbonic acid before settling back down in deep deposits.
The first living organisms on Earth were able to capture carbon and convert it into useful materials much faster than these slower geologic processes. Ever since then, carbon has been a key ingredient in all forms of life, so much so that the carbon cycle can be looked on as a mirror to the cycle of life itself.
The distribution of carbon between the earth’s crust, the oceans, the atmosphere, and the biosphere remained constant for millions of years. This all changed around 1750, the start of the Industrial Age, when humans realized that burning carbon-based fuels was a bountiful source of energy that could power all kinds of important manual and industrial activities.
Since then, roughly 5% of the 4000-6000 gigatonnes of all the carbon stored in Earth’s fossil fuel deposits have been burned, releasing roughly 280 gigatonnes of carbon into the atmosphere. Other important changes occurred too, like deforestation required for mass agriculture and industrial operations. This changed the way land was used and reduced the amount of vegetation capable of capturing atmospheric carbon, resulting in a further 150 gigatonnes of net carbon added to the atmosphere.
Taken together, these phenomena had the effect of doubling the amount of CO2 in the atmosphere to 780 gigatonnes, where it stands today. Contemporary estimates suggest that each year, a further 40 gigatonnes is added to the count.
That is definitely a lot of carbon, but it is a drop in the bucket compared to the amount of carbon stored in the deep ocean, which totals 38,000 gigatonnes. Even that is a tiny fraction of the carbon already naturally stored in the lithosphere in the form of sedimentary rocks or limestone, which equates to 50,000,000 gigatonnes or 5 quadrillion tons.
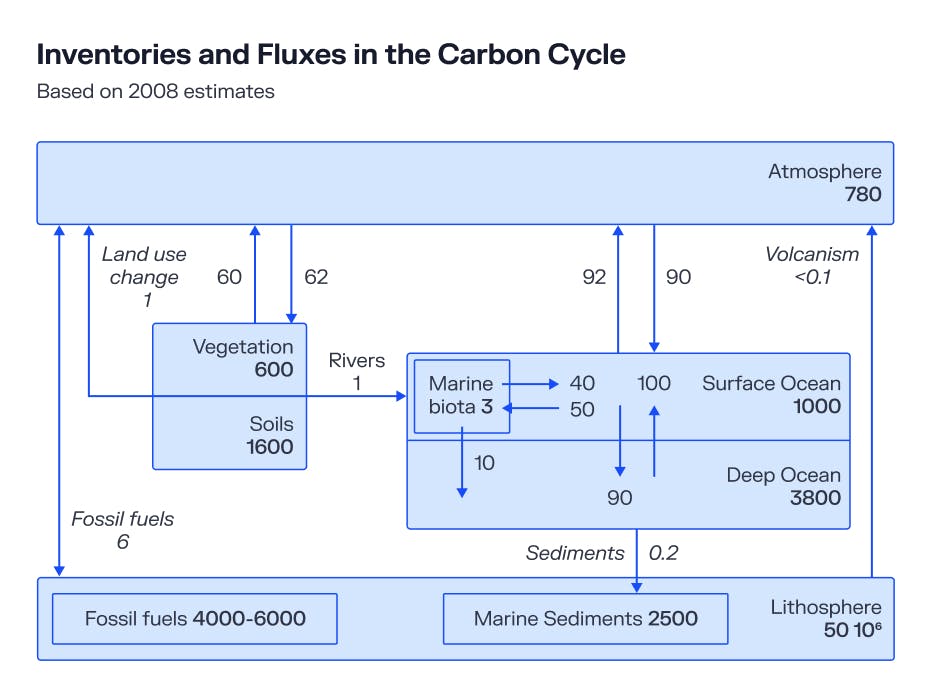
Source: Carbon Capture and Storage, Stephen A. Rackley
The sheer scale of carbon storage in these natural carbon sinks has given environmental scientists and engineers an idea — why not return the carbon back into the deposits from which they came? Therein lies the seed of the idea of geological or deep-sea carbon sequestration.
There are multiple operational projects across the United States, Canada, Australia, and Europe that are already storing carbon underground permanently in deep underground formations like saline aquifers or depleted oil or gas reservoirs. Other more esoteric ideas involve building geological features that could become passive carbon sinks over time, like this proposal to build a sponge mountain in Turin. Though these methods are effective at putting carbon away for good, there are some concerns about the environmental and safety risks associated with them. They’re also rather expensive. Outside of selling carbon credits for CO2 stored away, there are currently few economically viable pathways to store the carbon at scale.

Source: Dezeen
The Economics of Direct Air Capture
Just as the question of where to put sequestered carbon has no firm answer yet, there is not yet a set standard for how to actually perform direct air capture. Though a number of mechanisms do exist, the economics will decide which techniques are most scalable and endure over the long run.
In general, there are two steps necessary to conduct direct air capture. The first is acquiring a sorbent, or a material capable of attracting carbon dioxide molecules to itself. The second step involves distilling carbon or carbon dioxide from the sorbent.
Both of these steps require energy. In the first case, companies like Carbon Engineering or Skytree operate massive fans that pull in air and run it through aqueous sorbents like hydroxide solution (in the case of Carbon Engineering) or plastic resins which attract carbon dioxide (in the case of Skytree). The resulting solutions are then heated and reacted with another chemical to release carbon dioxide again in a pure state.
The energy involved in running the fans and heating the solutions is currently the biggest cost center associated with running direct air capture plants, which is why alternative direct air capture designs are looking to deal away with these steps entirely.
Klaus Lackner is now working on a system that can attract carbon dioxide-filled air passively via a solid-state sorbent. He stumbled upon a unique kind of resin that attracts CO2 when dry and releases it when wet. The prototype of this new technique is what Lackner is calling “Mechanical Trees.”

Source: Arizona State University
Mechanical trees are described by Lackner as follows:
“They’re tall vertical columns of discs coated with a chemical resin, about 5 feet in diameter, with the discs about 2 inches apart, like a stack of records. As the air blows through, the surfaces of the discs absorb CO2. After 20 minutes or so, the discs are full, and they sink into a barrel below. We send in water and steam to release the CO2 into a closed environment, and now we have a low-pressure mixture of water vapor and CO2. We can recover most of the heat that went into heating up the box, so the amount of energy needed for heating is quite small.”
Though the Mechanical Trees approach does away with fans and decreases the amount of heat required to release carbon dioxide, the jury is still out on whether passive and solid sorbent approaches like this are indeed the most economical. Notably, the team behind Carbon Engineering’s approach claim that “the challenges of solid sorbent designs are first, the need to build a very large structure at low cost while allowing the entire structure to be periodically sealed from the ambient air during the regeneration step when temperature, pressure, or humidity must be cycled. And second, the inherently conflicting demands of high sorbent performance, low cost, and long economic life in impure ambient air.”
What’s missing from both approaches, however, is scale. Climeworks’ largest plant in Iceland can only process 4,000 tons per year, while Carbon Engineering’s pilot facility in British Columbia can process less than 400 tons a year.
The high fixed costs associated with building direct air capture plants and the limited scale they can currently process naturally bring the per ton cost of carbon capture to prices exceeding economic viability today. However, Lackner believes that simply growing the capacity of the industry will be enough to reach more affordable targets. A similar pattern has occurred in both the solar panel and battery fields. As these industries grew, the additional capacity and learning rates associated with scaling eventually brought costs down tremendously. Lackner is convinced that growing the direct air capture industry by a factor of 300 will be enough to eventually reach a cost of $100/ton — a price at which many agree carbon removal efforts will finally become economically feasible.
The Economics of Carbon Utilization
Luckily, efforts to scale direct air capture are already in the works. One of Carbon Engineering’s forthcoming projects, located in Texas, is expected to begin operating in 2024 and will have enough capacity to remove 500,000 tons of carbon per year. It will eventually be scaled up to remove up to one million tons per year. Part of what’s enabling Carbon Engineering to build a plant this big is that the carbon they remove will have immediate utility.
Most of the carbon the Texas plant removes will be used for what’s called “enhanced oil recovery” – which is essentially a process where carbon dioxide is pushed underground to liberate more oil. Though this may sound counter-intuitive, enhanced oil recovery was one of the first industrial use cases for captured carbon, and currently constitutes the world’s biggest market for captured carbon.
To improve their economic viability, carbon removal companies are looking for a killer use case that can fuel their continued growth. Though enhanced oil recovery is the most viable option now, others are emerging.
Given that carbon is a ubiquitous component in most substances – from alcohol to lipids to fuels and plastics – there’s a lot that can be manufactured with carbon as a material input. The only problem is that carbon is pretty inert, which means it takes a lot of prodding to get it to react and form more complex molecules.
Currently, the most successful technique for converting carbon dioxide into other useful compounds is called electrocatalysis. It’s a process that uses charged catalysts like molecules or ions in addition to an electric current to get carbon to bond with atoms like hydrogen and form slightly larger molecules like methane (CH4) or ethylene (C2H2). The ins and outs of this process are still being researched heavily, and various labs across the country are investigating the most effective catalysts. Some have found that copper-based catalysts are really good at producing methane and ethylene, while others have noted the effectiveness of silver-based catalysts in producing carbon monoxide or formic acid. Other labs, like one at Cambridge University, are experimenting with even more creative catalysts like enzymes.
The problem with electrocatalysis is it’s very energy intensive, especially if required to run for a long time to produce some of the longer-chain carbon compounds. To remain carbon neutral, most operations like Carbon Engineering use renewable sources like solar energy or wind energy to supply the electricity required for electrocatalysis. Given the limited capacity of the plant and the high energy demands of the process, the most that Carbon Engineering can currently produce are single or two-carbon chain compounds like methane and ethylene.
Longer-chain carbon fuels are more valuable, as the longer the carbon chain the more energy there is stored in the molecule. However, the more energy a molecule has, the more energy is required to get that molecule to bind together in the first place. The holy grail of electrocatalysis would be a process that could directly convert carbon dioxide into long-chain carbon compounds like butane or petroleum, but because of energy constraints, it’s more efficient for a plant like Carbon Engineering to stop at methane and sell these compounds downstream to larger chemical processes that can use them in industrial applications.
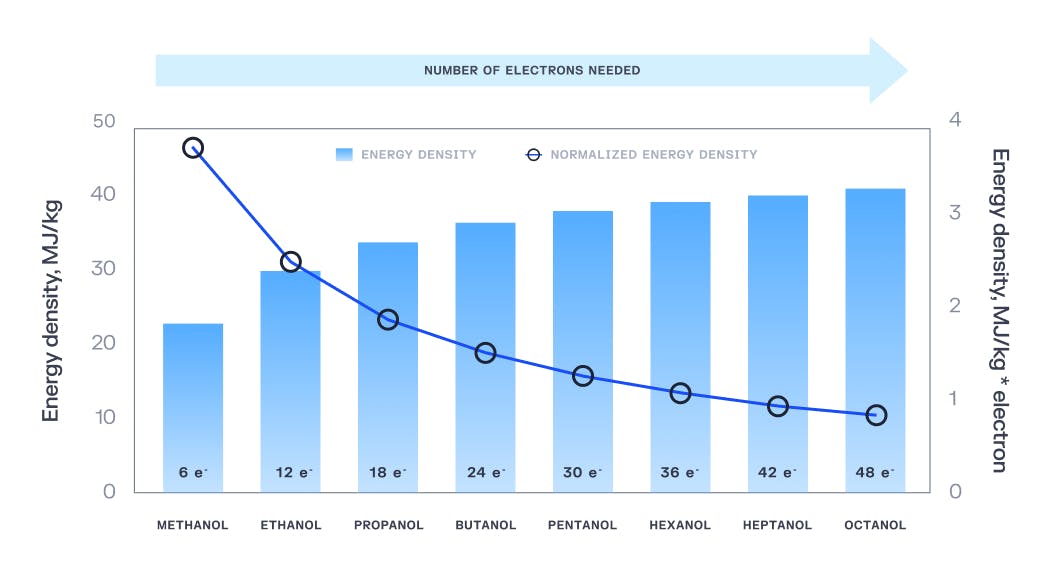
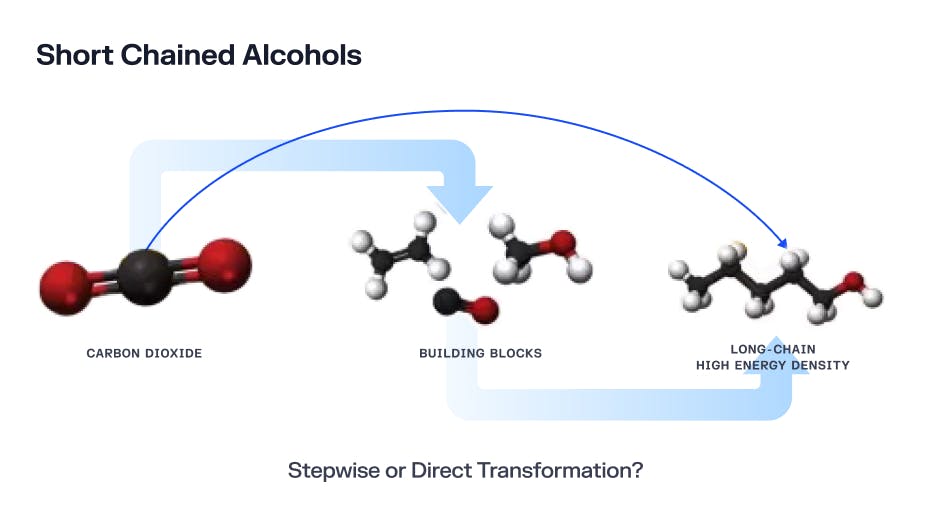
Source: What Should We Make with CO2 and How Can We Make It?
The Future of the Carbon Economy
Creating a cyclical economy of fuel combustion, emission extraction, and fuel production with carbon removal and utilization technologies would be an elegant solution to many of our climate problems. Still, these methods only amount to net neutral strategies that prevent further emissions but don’t help remove excess carbon already in the atmosphere at scale. There are few customers willing to pay companies to sequester carbon underground, but there could eventually be a real market for sequestering carbon in all kinds of products we use every day. Imagine if everything from tupperware to polyester pants were made of carbon extracted from the skies.
This would be a clever way of sequestering carbon into materials that could continue sustaining our material well-being on Earth while creating the economic demand necessary to extract carbon from the atmosphere at scale. However, the path to getting there is still filled with uncertainty.
One promising area of opportunity would be to produce widespread industrial materials like concrete using sequestered carbon emissions. Though there exist some commercial products produced with sequestered carbon, like ethanol (used in certain laundry detergents) and soda pop (where carbon is sequestered in our carbonated colas), the reality is that we’re still not very good at manufacturing stuff out of carbon.
But others are. In fact, plants do it all the time via photosynthesis. Photosynthesis is the mechanism that converts sunlight and carbon dioxide into energy-rich compounds like sugars and proteins. Remarkably, we still have a very limited understanding of how photosynthesis works. The few times we’ve tried to replicate it — artificially using sunlight and carbon dioxide to produce starches — the process ended up being extremely inefficient. However, the idea is pretty intriguing. The best attempt at replicating photosynthesis so far, conducted by scientists in China, used carbon dioxide gas to produce edible starches. Though it only achieved a 2% energy conversion rate, the idea of turning carbon dioxide into food could potentially become yet another carbon sequestration pathway.
More importantly though, if we could ‘grow’ plastic or carbon fibers as easily as plants grow their leaves, we could all breathe a de-carbonated sigh of relief. It’s not science fiction, this is one of many pathways under investigation in the realm of carbon compound production. As we search for new ways to produce novel materials in new ways, previously disparate fields like material science and climate engineering may find more and more common intersections.
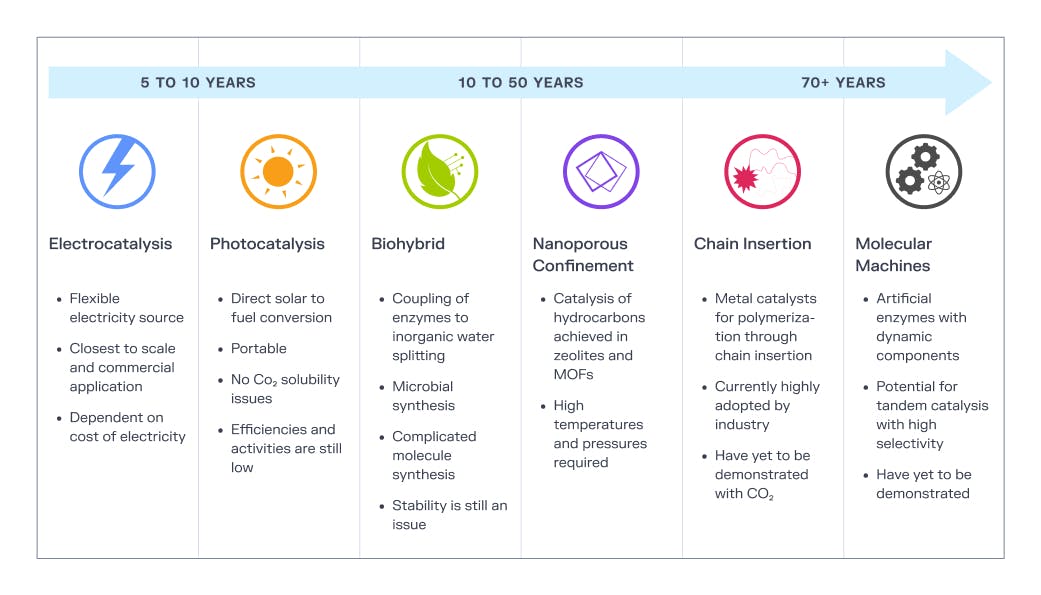
Source: What Should We Make with CO2 and How Can We Make It?
Whether it’s better technique and new chemical advancements that allow us to do more with carbon, or breakthroughs in physics that give us access to cheap and abundant energy, we’re moving towards an exciting future where the carbon in our atmosphere no longer has to be seen as a pollutant, but instead a resource.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly, Fund of Fund investments and investments in which total invested capital is no more than $50,000) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.



