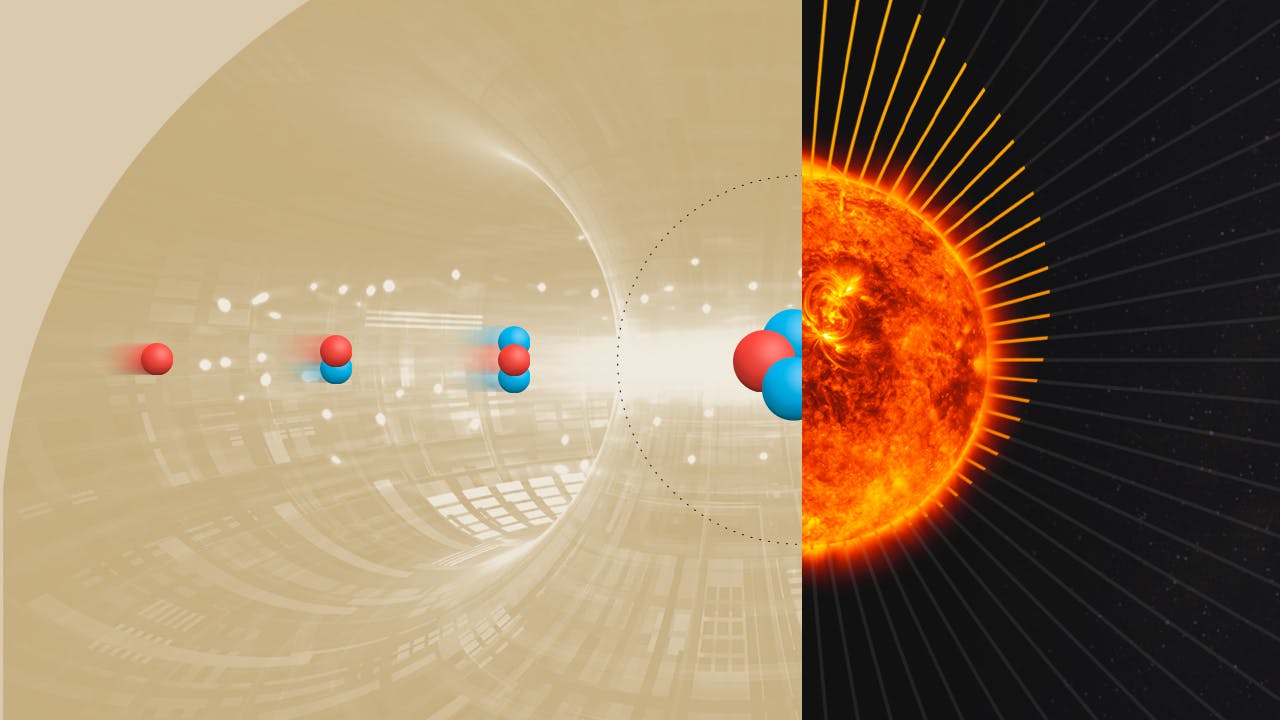
Fusion could be the most consequential technology ever produced by humanity. There’s a profound, almost mystical, symbolism in our ability to confine the power source of our solar system into a test tube. In some ways, achieving fusion will feel like a final triumph over nature. After millennia of staring up at the Sun and worshipping its role in giving energy to all life on Earth, we are now learning to mimic and reproduce this power for ourselves.
Our version of fusion, however, will arguably be even more efficient than the processes happening in the Sun, because it will need to happen faster, at higher temperatures, and in a smaller area than that available to the Sun. In fact, fusion on Earth will need to happen at temperatures ten times higher than those in the very core of the Sun, a fact that seems like it should be impossible.
However, that’s not the only thing that sounds impossible about fusion. It promises energy abundance, a near-infinite fuel supply, and the ability to fuse new elements and increase the power available to each person which could completely reshape human life on Earth. As hard as it is to fathom that we are now capable of developing technologies that produce tiny stars, it’s even harder to imagine what this technology will allow us to achieve in the decades and centuries to come.
For these far-reaching economic, but primarily security implications, fusion research has been kept secret for decades. It has been shielded under the umbrellas of national defense departments, given its close relation to efforts to build weapons of mass destruction, a security measure which has nonetheless delayed humanity’s ability to achieve energy abundance. Despite the years of secrecy, incredibly specialized knowledge required to understand it, and the prohibitive costs involved with studying it, the fusion space is now saturated with numerous private companies determined to find breakthroughs that help propel this technology to mass adoption as soon as possible.
The Current State of Fusion
For over a hundred years, we’ve been aware that when two hydrogen isotopes fuse together, an incredible amount of energy is released and a helium atom is produced. For over a hundred years, we’ve known that this reaction has been responsible for powering the Sun and every other star in the universe — and for just as long, scientific debate has raged about whether it would even be possible to replicate this same process on Earth.
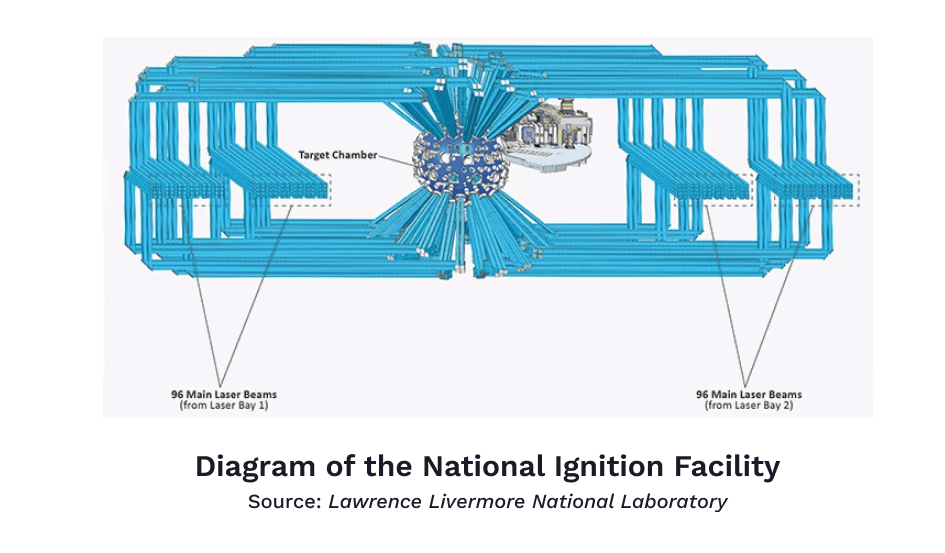
On December 5th, 2022 the National Ignition Facility in the Lawrence Livermore Laboratory laid all such doubts to rest. A multi-billion-dollar undertaking of the Department of Energy, home of all things nuclear in the United States, the National Ignition Facility is the largest and most powerful laser on Earth. Actually, it’s not just one laser — it’s 192 lasers lined up next to each other, spanning the length of two football fields. The light from these lasers is accelerated and amplified six times before being converted into ultraviolet light and targeted precisely onto a metal cavity that contains a tiny capsule filled with hydrogen isotopes. The lasers are shot at the capsule for a billionth of a second and produce a temperature ten times hotter than in the center of the Sun.
At about 100 million degrees Celsius, hydrogen isotopes are colliding into each other with such immense speed that they begin to fuse. As they do, they release a blast of energy so powerful it pushes other hydrogen isotopes to fuse, and so on, until all the fuel in the capsule is used up.
This process of heating up the capsule to the point that all its fuel is consumed in a fusion reaction is called ignition. The concept is nearly equivalent to the act of producing enough friction to light a match. Theoretically, it sounds simple enough, but practically, it has taken decades, billions of dollars, and hundreds of attempts to get right. For years, the NIF’s failed attempts led to it being ridiculed as the “Never Ignition Facility” or the “Nearly Ignition Facility”, so you can imagine the relief that ensued when the thirteen-year-old, $3.5 billion laser system finally managed to light fusion’s match. Upon hearing the news, Tammy Ma, who heads the fusion initiative and has worked at the Lawrence Livermore lab for over 14 years, burst into tears while waiting to board a plane.
Ultimately, the NIF laser beamed 2.05 megajoules of ultraviolet light at the fuel capsule, inducing a fusion reaction which released 3.15 megajoules of energy.
To be clear — 3.15 megajoules is not even one percent of what a nuclear power plant produces every hour. In fact, it’s just about the amount of energy required to boil two pots of water. However, the NIF was not built to be a functioning power plant, it was built as a laboratory experiment with the explicit purpose of achieving fusion ignition. That effort was a resounding success, and it has placed the coveted milestone of achieving ignition into our rearview mirror once and for all.
The coming challenge for the scientific community devoted to making fusion power a reality now lies in figuring out how to scale this process to an industrial level. Achieving feasible fusion power is a massive design problem which currently presents more questions than answers. Though to better understand all the possible paths forward, let’s review the history of our attempts to unlock star power on Earth.
Discovering Fusion
The secret behind the Sun’s endless power was always a deep, cosmic mystery for a long time. Ironically, it was a quest into the nature of the very small that unlocked insights into the very big.
In 1919, Francis Aston, a precocious British chemist, was determined to measure the weight of an atom. It was just a year after the end of the First World War, which had him enlisted in the army servicing some of Britain’s earliest air force fleet. The war had thwarted his desire to burrow away in a lab and focus exclusively on research. Finally, he had returned to Cambridge to conduct his experiments in peace. The early 20th century was an exciting time in chemistry. There was a firm understanding that all matter was composed of elements — particles of an essential nature, which could not be transformed from their fundamental state. Centuries of discovering solid elements like metals, and distilling various liquids and gases, resulted in humanity collecting a list of such fundamental elements. The list included things like gold, silver, sodium, oxygen, and hydrogen. Various forms of chemical analysis helped establish the approximate relative weights between the elements, which allowed Dmitry Mendeleev to organize them according to their mass in a table — today’s periodic table.
At the time, the properties of elements and their relationships were understood only approximately, but Aston was not satisfied with approximation. He wanted to measure the precise weights of the atoms themselves.
The way to do it was a clever trick, analogous to determining someone’s height by measuring their shadow. He figured that if he could run ionized atoms through a magnet that deflected the path of their flow, he could determine the weight of ions by the amount the magnet deflected them. The lightest atoms were easiest to deflect, and the heavier atoms were the hardest.
This device created to enable this measurement is known as a mass spectrometer, and it revealed something weird about the weights of the individual atoms. Aston found that the elements were not actually perfect multiples of hydrogen’s weight, they always weighed ever so slightly less than a perfect multiple of hydrogen. For example, helium wasn’t four times the weight of a hydrogen atom, it was actually 3.97 times its weight. This discrepancy carried a clue.
The results of Aston’s experiments made the rounds and caught the attention of an astronomer and physicist named Arthur Eddington. Eddington had read Albert Einstein’s 1905 paper years ago, which claimed that there was an equivalence between mass and energy — E=mc^2. The mystery of the missing mass could only mean one thing in Eddington’s mind — the conversion of mass into energy. Eddington was preoccupied with thoughts of stars, planets, and galaxies, and suddenly this insight from a chemist opened his eyes to the mechanism that makes the Sun shine.
Eddington announced his reflections from Aston’s experiment at a conference, saying:
“Aston has further shown conclusively that the mass of the helium atom is less than the sum of the masses of the four hydrogen atoms which enter into it and in this at least the chemists agree with him. There is a loss of mass in the synthesis amounting to 1 part in 120, the atomic weight of hydrogen being 1.008 and that of helium just 4.00. . . . Now mass cannot be annihilated and the deficit can only represent the mass of the electrical energy liberated when helium is made out of hydrogen. If 5% of a star’s mass consists initially of hydrogen atoms, which are gradually being combined to form more complex elements, the total heat liberated will more than suffice for our demands, and we need look no further for the source of a star’s energy.”
The year was 1920. A hunch had clued Eddington in to the fact that the fusion of hydrogen atoms into more complex elements was secretly powering the Sun. It was a guess that predated the existence of quantum mechanics, and even predated our understanding of the atomic structure — but it was basically spot on. The only thing Eddington got wrong was the assumption that only 5% of the Sun’s mass consisted of hydrogen. It was actually 100%.
The dream of fusion power on Earth was born then and there. "If, indeed, the subatomic energy in the stars is being freely used to maintain their furnaces, it seems to bring a little nearer to fulfillment our dream of controlling this latent power for the well-being of the human race — or for its suicide,” said Eddington.
The discovery of this process was the first time that scientists stopped looking at atomic elements as rigid and unchanging and began looking at them as dynamic particles in the universe, capable of being fused or broken up into smaller parts under the right circumstances. It was also the first time they realized just how much energy was locked inside even the smallest of particles.
Measuring the weights of all the atomic elements revealed an interesting trend. Atoms lighter than iron weighed progressively less than the sum of their constituent particles. It seemed that whenever lighter atoms fused to form a heavier element, energy was released. However, that pattern only held for atoms lighter than iron.
Atoms heavier than iron needed energy injected to help them fuse. Elements as heavy as uranium, with 92 protons and anywhere from 141 to 146 neutrons, were so heavy they naturally wanted to come apart. These atoms were so unstable, they became radioactive, releasing energy as protons and neutrons naturally broke away from the precarious core.
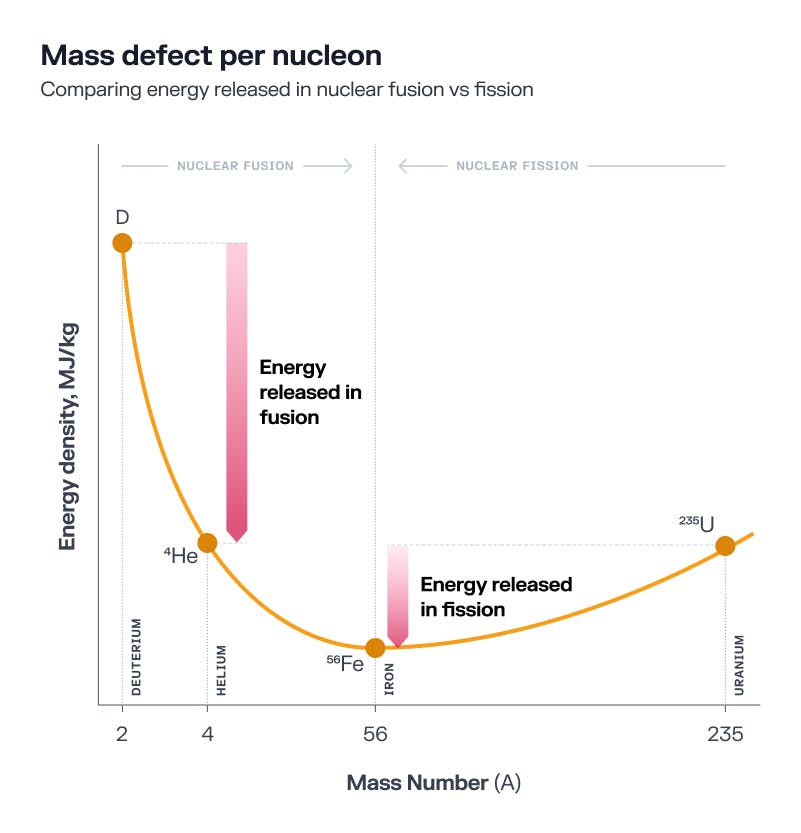
Source: Fusion: The Energy of the Universe
It was clear that way more energy was released when two small atoms fused than when one heavy atom fissioned. However, the activation energy required to get an already heavy and radioactive element to break apart was way lower than the energy required to force two hydrogen atoms to fuse into helium. Doing that would require conditions as hot and dense as in the center of the Sun.
From Theory to Practice
In the core of the Sun, temperatures reach 10 million degrees Celsius. At this temperature, the atoms get so hot, they form a plasma. Plasma is basically a gas that has become so hot, its electrons start roaming around freely and the entire plasma starts conducting electricity.
The process of fusion begins with two protons. Protons are positively charged and, under normal circumstances, repel each other. However, inside a hot plasma, particles are flying into each other so fast that they overcome electromagnetic repulsion and instead fuse together. Once this happens, one of the proton becomes a neutron and together, they form an atom called deuterium, which is really just a hydrogen atom, with an extra neutron. The resulting deuterium is then involved in a second fusion reaction. It actually fuses again with more deuterium, or with tritium — hydrogen but with two extra neutrons — to produce helium.
The energy released during the constant fusing of hydrogen atoms creates enormous outward pressure that is 400 billion times higher than the atmospheric pressure on Earth. However, due to the Sun’s enormous mass, its gravity is strong enough to counteract the outward pressure and keep the hot, fusing plasma compact in a sphere.
Trying to recreate the Sun on Earth was a good first blueprint for designing a fusion plant. In theory, the concept was very clear — heat hydrogen gas to the point that it becomes a plasma, wait until the hydrogen atoms start fusing, and collect the energy produced. The fusion between deuterium and tritium requires less energy to initiate than the fusion of two protons, so this reaction became the focus of fusion efforts on Earth. George Thomson, whose father J. J. Thomson discovered the electron, was the first to propose an idea for a fusion plant along these principles.
The only problem here is that plasma is extremely unstable. In the Sun, the explosive energy released by fusion reactions is contained by the Sun’s massive gravitational force. On Earth, the task of stabilizing an exploding, hot plasma has been compared to “holding jelly with elastic bands.”
It’s important to keep the plasma stabilized during the course of the fusion reaction so that all of the fuel in the plasma can be used. Otherwise, an unstable fusion reaction would just blow the plasma up and spew perfectly good hydrogen fuel all over the place. More importantly though, the hot plasma would instantly melt any part of the reactor it physically touched.
One approach to solving this problem was by taking advantage of the fact that plasma conducted electricity. The laws of electromagnetism state that every electric field induces a magnetic field, and vice versa. So, a sufficiently powerful magnetic field interacting with the plasma might be able to control its shape and keep it contained. This approach was called magnetic confinement but in practice over the past fifty years, holding plasma together for a long enough time to induce a protracted fusion reaction has been unsuccessful.

Having failed to realize fusion via magnetic confinement for many years, around the 1970s, physicists began looking for other approaches. Rather than heating a plasma, and stabilizing it while all its hydrogen atoms fused, they wondered if you could induce a small fusion reaction so fast that you didn’t have to deal with the problem of holding the plasma together. The amount of fuel used would be smaller, but the reaction would happen so quickly that all the fuel would instantly be used up. The inaugural paper for this approach called inertial confinement was co-authored by John Nuckolls in 1972. Years later, Nuckolls became the director of the Lawrence Livermore laboratory, which started building experimental equipment to test this new approach. Planning for the National Ignition Facility began under Nuckolls’ directorship in the early 1990s, but ground was only broken in 1997, and the facility didn’t become operational until 2009. This excruciatingly slow timeline is a telling detail that lends partial insight into why fusion has taken this long to mature. Finally, fusion ignition was achieved on Earth. It did not happen by magnetic confinement, but by inertial confinement, in the same lab where the approach was initially developed, some fifty years earlier.
The Challenges of Mass Production
Fusion is one of the only means of generating energy where abundance of fuel is not a concern. Unlike wood, coal, natural gas, or even uranium, which are all limited in supply — deuterium and tritium are very easily obtained. Deuterium is just an isotope of hydrogen, and roughly one in every 7000 hydrogen atoms is exactly the deuterium isotope we need. Deuterium can easily be distilled from water, and tritium, the hydrogen isotope with two neutrons, can be produced by reacting neutrons with lithium in a fission reaction.
However, whereas igniting fuels like wood or coal is pretty straightforward, the process of igniting hydrogen isotopes has taken decades. Now that we’ve finally achieved the milestone of ignition, the work begins to build a fusion plant that can generate enough energy to power a city.
Though we’re far out from that reality, here is the general idea of how a large-scale fusion plant should run:
- Deuterium and tritium fuel would be heated from an external energy source until they fused. The fusion reaction would produce a helium nucleus and a neutron. 80% of the energy released during the reaction would be carried by the neutron, and the other 20% would be carried by the helium nucleus.
- Helium nuclei are stripped of their electrons, and thus have a positive charge. When produced by the fusion reaction, they would be trapped by the magnetic field surrounding the reaction, and their energy would be used to heat the plasma further and ignite more fusion reactions.
- Eventually, more and more of these trapped helium nuclei would provide the energy required to continue the fusion reaction until the entire reaction becomes self-sustaining, which is the goal of ignition.
- The neutron carrying 80% of the reaction’s energy lacks any electric charge, and would not be confined by the magnetic field. It would fly outward, into a surrounding structure containing lithium, there it would react in a fission reaction with the lithium to produce more tritium fuel and generate a great amount of heat.
- The heat given off would boil water, which would produce steam and power a turbine that would generate electricity, just like any major power plant.
Though we have a good sense of how the picture should work as a whole, many details are still unresolved and pose massive engineering challenges for the coming generation of physicists and engineers. These are some of the most immediate problems that would need to be solved provided we stick with the inertial confinement approach:
- Finding a repeatable way to heat the fuel capsule. The external source of energy used to heat the fuel capsule is called the driver. The National Ignition Facility used lasers as their driver to heat the fuel. However, the NIF’s lasers can only be fired once a day, since doing it more often would damage them. This is pretty problematic since a fusion plant using the inertial confinement method would need to drive the reaction something like 10 times every second.
- Finding an energy-efficient way to power the driver. The NIF’s laser requires 300 megajoules of energy to power a 2-megajoule laser blast, which means their driver system converts only 1% of its energy into heating the target. The 2-megajoule blast is capable of igniting a 3-megajoule fusion reaction, but relative to the energy required to power the laser in the first place, the total fusion reaction in the laboratory comes to a massive big energy loss.
- Designing a reactor structure that could withstand the effects of neutron bombardment. The neutrons flying out the reaction chamber slam into surrounding walls, putting great strain on the reactor walls and structure over time. A feasible reactor would need to withstand this kind of bombardment for years while maintaining its structural integrity. Over time, the reactor structure would become radioactive, with its internal atoms being destabilized by the neutrons. At the end of the reactor’s lifetime, the structure would need to be sealed to protect from its radioactive effects.
There are dozens of private companies working on solving these problems. Some, like First Light Fusion, are developing an entirely new type of driver system, which would heat the fuel capsule by blasting it with ions at incredibly high speeds. They’re calling this amended version of inertial confinement, ‘projectile fusion.’
It’s unclear, though, if inertial confinement can be successfully commercialized. It could be that a completely new direction might prove more practical in the long run. The nine-year-old startup Helion, for instance, has developed a unique reactor design around the principles of both magnetic and inertial confinement. On either end of their seventh generation “Trenta” reactor, two symmetrical discs of hot plasma are generated before being slammed into each other and compressed by strong magnets to induce a fusion reaction.
The most interesting part of Helion’s design is that its reactor can generate electricity directly from the reaction. When fusion begins to occur and the plasma starts creating outward pressure, it causes changes in the magnetic field which can be directly recaptured as electricity. This way, no water has to be boiled, and no steam-powered turbines are required to generate electricity. Though these are the traditional methods every major power plant uses, Helion has brilliantly designed a mechanism that reimagines what a large-scale fusion reactor could be capable of. It’s due to complete building its 8th generation reactor, “Polaris,” in 2024, which should be capable of converting fusion energy directly into electricity.
Despite these incredible efforts, it’s not obvious when fusion will be ready for commercial use. Extreme optimists say we’re only about a decade away, while skeptics say we could be waiting fifty years or more. Whenever fusion power comes online, there’s no telling what a fusion-enabled society will look like. The promises of abundant energy and nearly infinite stores of fuel could open up the feasibility of everything from inter-planetary travel to developing a functioning artificial general intelligence. The only thing that’s certain is that the cone of possibilities for humanity will widen immensely.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly, Fund of Fund investments and investments in which total invested capital is no more than $50,000) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.