Metallurgy is really modern day alchemy. It's sometimes mesmerizing to stare at your laptop and realize that most of the materials used to make it were once rocks.
Of course, it's not just our laptops. Every important economic activity, whether it's construction, transportation, electrification or computation relies on metal. No other material has this amount of useful properties. Metal can be strong yet light, durable yet malleable — it can even conduct electricity. The more we discovered about the properties of metals, the more types of technologies we were able to build. In fact, the history of metals very closely traces the history of modern technology.
However, the story isn't over for metals. We're not done figuring out how to use them nor are we done figuring out how to make them. Our reliance on them is a bit of a double-edged sword. We need them for everything, but metals are also a finite material.
Some estimates suggest that in a few decades, the global demand for steel, aluminum and copper will be three times what it is today, which will be a real challenge for the industry since over time, mining new metal or recycling used metal will only get more challenging, and new strategies will be needed to continue supplying our economies with the materials they need. Even if common metals like steel and aluminum are relatively abundant, modern devices like our phones feature nearly a third of all the elements on the periodic table, and a lot of those elements are more scarce than we'd like to admit.
We're entering an interesting new era in the world of metal production, where our most crucial industries and manufacturing processes will need to evolve drastically in an environment of growing material uncertainty. It's why there's no better time than now to trace the history of how we learned to work with metals, and become better acquainted with the techniques we'll need to address the challenges of the future.
The Origins of Metallurgy
The first metals were likely discovered by accident in prehistoric times. Nuggets of pure gold are known to have been lying in river beds all across the world, having been found in places as far flung as the waterways of present-day California to those of Egypt and the greater Middle East. Likewise, deposits of silver, copper, tin, lead and iron were discovered early on in their pure state.
The quantities of naturally occurring pure metals were limited though, and without knowledge of processing techniques, metals were originally mostly used for decorative purposes. In their pure form, gold, silver and copper and soft are pliable — useful for making jewelry, but terrible for use in armor or as weapons.
Most often, metals are found fused with other elements, like oxygen, in the form of ores. These ores are often colorful and eye-catching, so it's no wonder that prehistoric man was interested in collecting these rock ores too. Somewhere between 5,000 and 6,000 years ago, whether by accident or experimentation, metal ores were dropped into fire.

Applying heat broke the chemical bonds between the metal and the other elements making up the ores, transforming the ore from crumbly rock into a distillation of pure metal. Heating an ore to unlock its hidden metal, also known as smelting, was the earliest technique of metal extraction discovered, and it launched the millennia-old craft of metallurgy.
Smelting allowed early civilizations to produce larger quantities of metal. By throwing ores into hot kilns, collecting the resulting metals and fashioning them into useful forms, it became possible for early civilizations to manufacture instruments, weapons and sturdy vessels like copper pots or lead pipes used to carry water in the Roman Empire.
Further experimentation revealed that separate metals could be mixed together when melted. Bronze was the first alloy ever produced, created from a combination of copper mixed with tin. Alloys, it turned out, could have entirely different properties than their constituent ingredients. Instead of being soft like pure copper or tin, bronze was strong. The first durable swords, daggers and nails were made from bronze.
The adoption of bronze reached a natural limit, however, due to the scarcity of tin. In its place, another metal, initially known as a 'gift of the gods' soon took over the world.
Today, iron is looked upon as pretty unremarkable, but thousands of years ago, it was seen as a cosmic miracle. It was first discovered in its pure state by ancient Egyptians and Mesopotamians after it fell from the skies, carried to earth by massive meteorites.
Earthly iron, though, is not usually found in a pure state, which explains why it took longer to develop than other metals. Normally, iron is contained in iron ore, a granular and reddish compound of iron and oxygen atoms, better known to us as rust.
To extract it, iron ore needed to be smelted. Unlike copper, iron had a much higher melting point, so smelting it required hotter furnaces which burned for longer. The early attempts to refine iron were pretty disappointing. Pure iron was a lot weaker than bronze, but rumor had it that master metal workers who were around iron for longer, like the Assyrians as well as the Chinese and Indians, had figured out ways to turn this apparently soft metal into legendarily strong swords. It would take the rest of the world hundreds of years to approach the clever techniques used by ancient Chinese and Indian metallurgists. Everything from cooling the metal quickly, repeatedly heating it, folding it, beating it, heating for longer was tried. Though early metal workers didn't know why these techniques tended to work, the best ones were passed down with time and taught.
Mass Production of Metals
As populations and demand for iron grew during the medieval period, production needed to ramp up to keep pace. The limiting factor to smelting more ore was the ability to create a fire that was bigger, hotter, and could burn for longer.
A fire needs two things — fuel and oxygen. Early smelting furnaces, built with bricks to look something like a chimney, created an interior structure that contained the ore, fuel, and preserved the fire's heat. To introduce oxygen, hand-held bellows were used to blow more air in and keep the fire going. Designing a scaled up smelting furnace was tricky because even if you made these furnaces larger to accommodate more ore and more fuel, you would still need to solve for getting more oxygen to flow through them. A hand-held bellow just wouldn't do the trick.
In the 12th century, Europeans had developed a brilliant design for a smelting furnace that solved this problem. Unbeknownst to them, this technique was already standard practice in China for hundreds of years, but information traveled very slowly back then. Nonetheless, European metal workers were very proud to have independently figured out the 'blast furnace.'
Rather than use human labor to operate the bellows, a blast furnace relied on the awesome power of nature. Furnaces were built close to waterways so giant water wheels could mechanically operate bellows which would blast huge gusts of air into the furnaces to keep the fires roaring.
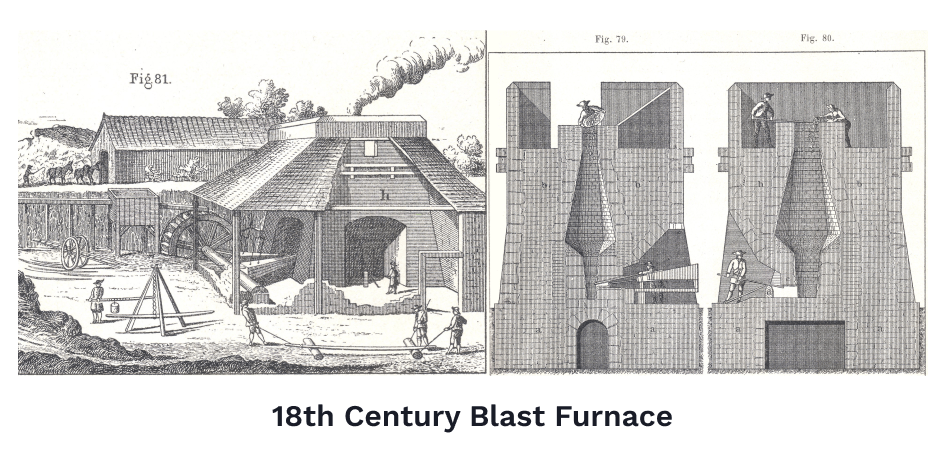
Blast furnaces could process a great amount of iron ore, getting the iron so hot it would melt. The problem was, when the melted iron cooled, it came out tough but very brittle. When whacked with enough force, it would just crack. Molten iron that came out of these blast furnaces would typically need to be poured into troughs to cool, and then worked to be made durable again.
What medieval metallurgists didn't realize was they weren't really in the business of making pure iron as they thought. All these years, they had actually been producing an iron alloy. Inside the furnaces, those big gusts of fresh oxygen were actually fusing with the carbon in the fuel to produce carbon dioxide, which exited through the top of the furnace. The remainder of the carbon in the fuel, however, mixed in with the molten iron. The substance that was being collected in those cooling troughs was an iron-carbon alloy, better known in the present day as steel.
Steel is a fickle beast. You need to hit a delicate iron-carbon ratio to produce a strong steel. Too much carbon, and the steel becomes tough and brittle. Too little of it, and the steel is soft and pliant. Getting the ratio just right was a headache for centuries and required tons of working and re-working either to get excess carbon out, or to get more carbon in.
It was only in the 19th century that a better solution was devised. An Englishman, named Henry Bessemmer, realized that traditional blast furnaces were introducing way too much carbon into the molten iron. To get it out, he suggested blasting the molten iron with even more oxygen. The extra oxygen would fuse with excess carbon and escape as CO2, leaving behind a low concentration iron-carbon alloy.
This technique became known as the Bessemmer process, and it single-handedly made the production of steel cheaper and more plentiful. If good strong steel normally took weeks to make, the Bessemmer process accomplished it in minutes.
Soon, steel was everywhere.
The Era of Cheap Metals
When Andrew Carnegie imported the Bessemer process to America, not only did it make him a magnate, it gave birth to industrial America. Cheap steel changed everything, and became an essential material in building the modern world as we know it today.
With steel, railways could be built long enough to span continents, connecting disparate markets, people and ideas which had previously been isolated. As a building material, steel proved so strong it eventually became a critical component in all forms of construction, allowing for the creation of the first skyscrapers.
Steel was also used to reinforce bridges, dams and roads. This meant more surface area for the burgeoning automotive industry, which itself drew heavily on steel as it became crucial to developing powerful engines that could withstand the high pressures of combustion reactions. Finally, in 1912, chromium was added to steel to produce a corrosion-resistant alloy known as stainless steel, which virtually all domestic appliances are made from today.
We're still living in the age of steel. As Vaclav Smil, author of Still the Iron Age puts it, "Steel is truly a ubiquitous material: there is no industrial enterprise that would not, directly or indirectly, rely on it. There is no modern construction activity that can proceed without it ... There is no commercial or household activity that would not, or ultimately, owe its existence to it."
Besides steel, the other metal most responsible for the creation of the modern world was the advent of cheap aluminum. Aluminum ore was first discovered in 1823, but it couldn't just be smelted. Throwing aluminum oxide into a hot fire would do nothing. It would take another half century to figure out how to separate aluminum atoms from oxygen, but eventually an American, Charles Hall, and a Frenchman, Paul Héroult, would solve the problem independently in the same year.
The basic idea was to dissolve the aluminum oxide into a solution — kind of like how salt dissolves in water — then, use a powerful electric current to separate positively charged aluminum ions away from negatively charged oxygen ions, a technique known as electrolysis. A lot of innovations in chemistry and electricity had to come first, but eventually, this process, called the Hall-Héroult Process, soon became standard practice in refining aluminum.
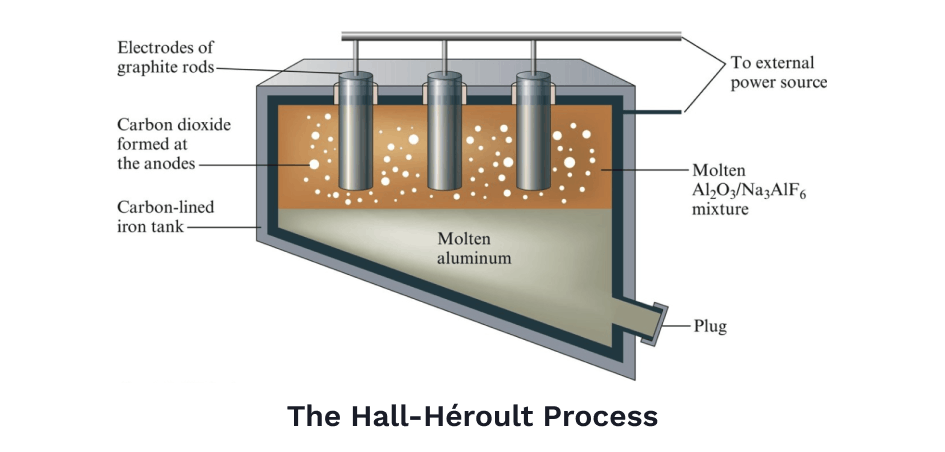
Before the Hall-Héroult process, pure aluminum was so rare, it was more expensive than gold. Napoleon Bonaparte was particularly fond of aluminum when it was first discovered. It was a marvelous metal — extremely strong yet light at the same time. Napoleon wanted the entire French army's armor made of the stuff. Though when that proved too expensive, he settled for making tableware out of it, serving only his most esteemed guests on plates of pure aluminum.
The going rate of aluminum around 1852 was around $1,200 per kilogram, but after the Hall-Héroult innovation, the price fell to $1 per kilogram by 1900. Once a material fit only for kings and emperors soon became a common kitchen occurrence which today, nearly every American uses to wrap last night's leftovers.
Aluminum's remarkably high strength to weight ratio transformed the world of transportation. Aluminum made train cars lighter, yet just as strong. Suddenly, engines were pushing a lighter load, which made trains faster. Aluminum made cars lighter and faster too, but most importantly, it allowed us to fly. Steel engines were just too heavy to propel airplanes, so with the emergence of a cheap alternative, the Wright Brothers designed their own engine made entirely out of lighter aluminum. In 1903, their aluminum engine allowed them to fly a controlled aerial vehicle for the first time in history.
Today, we produce over two billion tons of pure metal each year, and mine over three billion tons of ore to do it. The vast majority of the metal produced today is steel (at 1.95 billion tons per year), with aluminum at a very distant second (at 70 million tons per year). All the other metals on the periodic table barely even register when compared to the scale of the global steel industry today.
In total, the modern metals market is worth roughly $4 trillion globally, though this is likely to grow given the pressures of a growing world population, further industrialization, urbanization and digitization. By 2050, some estimate that global demand for steel and aluminum will grow up to three times today's output.
The Era of Material Invention
Over just the past 200 years, metallurgy has evolved from an artisan craft, relying on inherited techniques to a large-scale industry that draws on cutting-edge science to optimize production.
With breakthroughs in modern computing and physics, modern day metallurgists can control the composition of alloys with extreme precision and observe the internal structures of metal at the atomic level. They can even engineer metallic microstructures to achieve the exact structural characteristics that are desired. We're past merely discovering new metals. We're entered an era of unprecedented material invention.
The tools and machines we operate on a daily basis are now produced with hundreds of unique types of alloys. Digital devices feature up to a third of all the elements in the periodic table — and research is ongoing to produce newer, useful types of alloys.
These include new metal mixes that would make our car engines lighter, medical implants more resistant to corrosion, and the disposal of nuclear waste safer. New alloys are also in high demand in the aerospace industry, which needs to make rocket engines out of metals that can withstand tremendous heat. For example, SpaceX employs an in-house metallurgy team to engineer the uniquely powerful superalloy steels from which its Raptor Engines are cast.
The Era of Sustainability
Outside of the advanced techniques used by specialist metallurgists, most metal production processes have not strayed too far from those employed in the 19th century. As a result, the metal industry is one of the most energy-consumptive on Earth.
8% of the world's annual energy use is consumed via metal production. Roughly 75% of that energy goes toward making steel, while most of the remaining ~25% is devoted to producing aluminum. The process of electrolysis involved in the Hall-Héroult process is extremely energy intensive. Currently, one ton of aluminum is roughly six times more energy intensive to produce than a ton of steel. Overall, metal production accounts for over 30% of all annual greenhouse gas emissions.
To compound the issue, ore grades, which denote the concentration of a desired element in an ore, are decreasing so more ore is required over time to yield the same amount of metal. As our annual demand for metals grows, many are searching for ways to make current processes cleaner and more economical.
One solution that would address both issues is relying more heavily on scrap or recycled metals in the production process. After all, metals are infinitely recyclable. Throwing them in a landfill at the end of their lives is ridiculously wasteful. It's like re-burying something you spent a ton of effort trying to dig up in the first place.
Most don't realize it, but the metal industry already has a pretty big circular economy going, even if it's not an entirely closed-loop system. In fact, Coors was one of the first companies to figure this out way back in 1959, when they launched a recycling program for their aluminum cans. Coors would buy back empty cans to melt down and re-shape into their next batch. That way, a few grams of refined aluminum could produce an infinite number of cans in the future, decreasing material and production costs.
Today, nearly all shrewd manufacturers are following suit. After Boeing stamps large sheets of aluminum to fashion into planes, it sells off the leftover scraps to downstream recyclers who play a role in refining the aluminum into reusable sheets all over again.
Over half of the metal goods we use each day are made of recycled metals. Each year, between 80% and 90% of all discarded steel in the United States is recycled. Nearly all cars, for instance, are recycled for scrap. Steel beams that used to make up old buildings or bridges are transformed into future screws and hammers. Old fridges and microwaves are broken down and made into cell towers or engines. Even hardware producers like Apple have plans to re-engineer their manufacturing process such that 100% of the metals used in production come from recycled sources.
Recycling scrap metal makes metal production remarkably cheaper, especially when dealing with metals that require a lot of energy to refine — like aluminum. Producing ready aluminum out of scrap uses 16 times less energy than making it out of ore, since you’re circumventing the process of electrolysis altogether.
One of the forthcoming challenges will be developing and managing a system that can effectively recover metal products at the end of their lives. But even with effective recovery of metals, we would still need to figure out how to sort the different types of metals we recycle. The proliferation of more and more complicated types of alloys makes this a very hard problem and un-alloying all the alloys could actually prove to be very expensive.
Extremely sophisticated techniques are required to ascertain the precise composition of elements in an alloy. Everything from expensive X-Ray fluorescence machines to optical emission spectroscopy devices are used to deduce the exact chemical composition of metals in an alloy.
Successfully and cheaply separating out important metals will be a game changer in an environment where everyone is scrambling to secure a reliable supply of metals — particularly, rare metals which essential for batteries and digital devices, and are typically found in low concentrations.
Some researchers are also concerned that without diligent recycling measures, the metals in circulation might possess more and more foreign alloying elements which would alter their inherent structural characteristics — putting more pressure on engineers and designers to work around these defects.
Valuable materials aren't just found in mines anymore. Modern landfills are themselves veritable gold mines — today’s landfills often have more gold than literal gold mines. Whereas 200 tons of gold ore yield an average of one kilogram of pure gold, only 3-4 tons worth of circuit boards are needed to yield the same amount.
The growing emphasis on recycling and the management of new material flows is a new chapter being written in the evolving history of metallurgy. A story that began with swords being fashioned out of solid rust now finds itself in a phase where cars can be fashioned out of soda cans. The story of metallurgy and metal use, however, will only continue to change as it encounters new, more challenging constraints.
Some have called upon decreasing our dependence on metals as construction materials, but it's difficult to imagine any material that could realistically take their place. Given that metals will remain fundamental to our economy, the ways we create and use them is a story that will continue being written in the years to come.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly, Fund of Fund investments and investments in which total invested capital is no more than $50,000) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.



