Batteries are some of the most overlooked technologies. Today, they’re hidden behind the screens or user interfaces of the machines they power which obscure the essential role they played in electrifying human civilization.
Many forget that batteries were our first source of electricity, before we ever had access to or plans to build massive electrical infrastructure. In fact, we had batteries before we even knew what electricity was. Since the early 1800s, when the first battery was invented, the ability to store more power for longer has transformed our world. Better batteries made things like smartphones and laptops possible, not to mention other achievements like long-range, ultra fast electric vehicles.
Fully electrifying our infrastructure through renewable sources is one of the proposed solutions to reducing our reliance on fossil fuels. Unfortunately, in these debates, the role of batteries is often glossed over entirely. Batteries pose something of an uncomfortable conundrum here, since they too are made of finite materials and are not easily recyclable. The reality is that better battery technology might ultimately become the main bottleneck in achieving sustainable and abundant energy, which is why efforts to study them and eliminate their current limitations are so important.
The Latest in Battery Tech
The best battery on the market today is the lithium-ion battery. Developed in the late 1990s by Sony and the Asahi Chemical Company, lithium-ion batteries have become the ubiquitous de facto power source of electrical devices. More so than any other battery invented before them, they are incredibly energy dense, rechargeable with long life cycles, and since their invention have been falling precipitously in price. This suite of convenience characteristics is almost single-handedly what enabled our generation to have access to portable and powerful electronic devices.
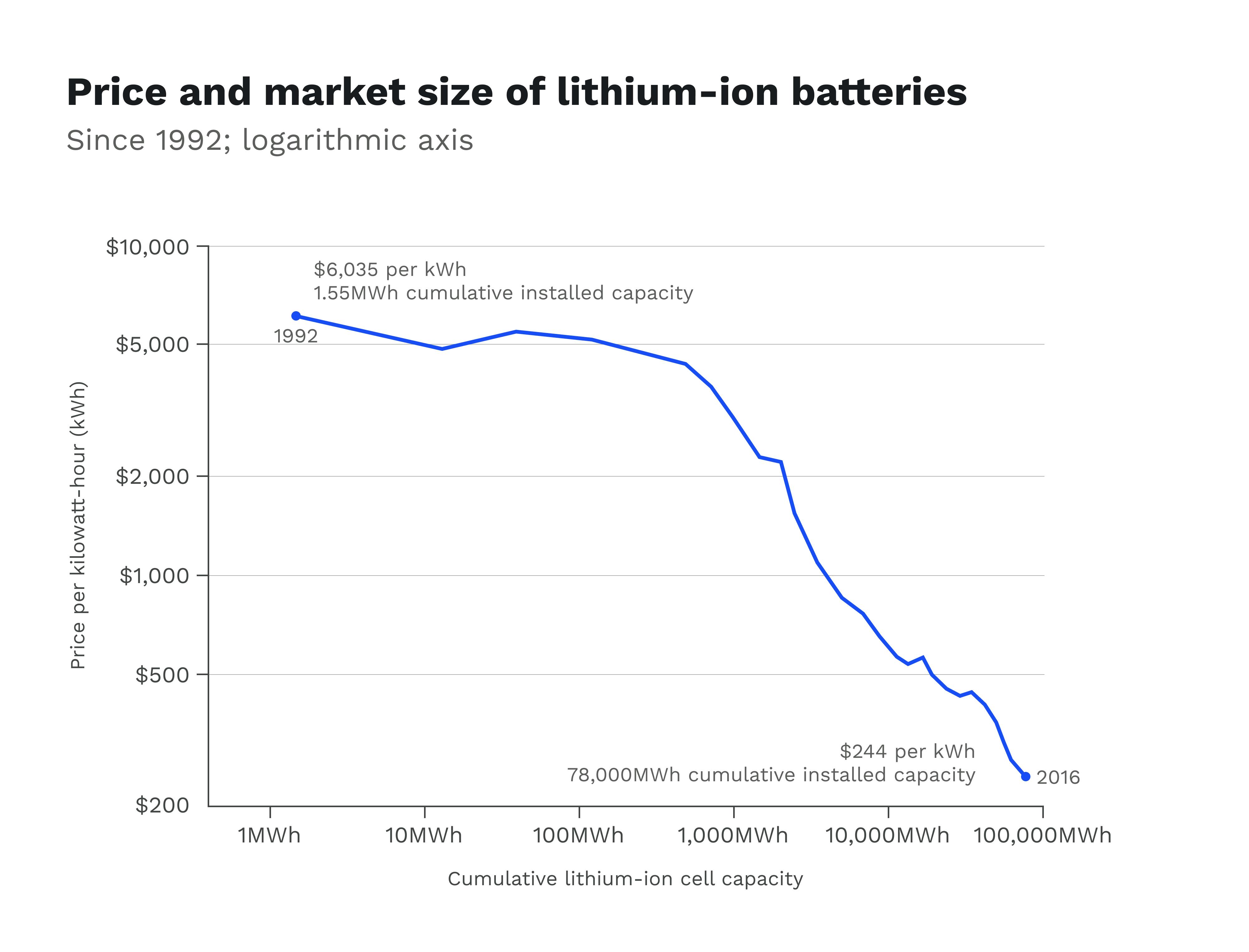
Source: Energy & Environmental Science
Nothing shows the full capability of lithium-ion batteries better than today’s electric vehicles. When the Tesla Roadster was unveiled in 2006 by Martin Eberhard, it was the first electric vehicle on the market that used lithium-ion batteries. At the time, other electric or hybrid brands like the Prius were using a nickel-metal hydride chemistry which had shorter life cycles and weighed more. Tesla’s initial design packed 6,831 individual lithium-ion battery cells — which look like regular Duracells on the outside — together to produce a 900-pound system that could bring the car from 0-60 mph in just four seconds. Today, using similar lithium-ion technology, Teslas can accelerate from 0-60 in just 2.3 seconds — making them the fastest cars in the world.
And there’s still more juice to be squeezed out of lithium-ion technology. Panasonic is one of the most advanced battery manufacturers in the world and has been cooperating with Tesla on battery design basically since Tesla’s inception. They’re now inches away from releasing a still more energy dense lithium-ion cell, which will be able to store 900 watt-hours per liter over the current top-of-the-line battery, which has a maximum energy density of 750 watt hours per liter.
While this would be an impressive improvement, there is a sense that lithium-ion batteries in their current design are entering a world of incremental gains.
The Battery Matrix
Ultimately, batteries are evaluated on five characteristics:
- Energy density — a measure of how much total charge a battery can store
- Power density — a measure of how the maximum power a battery can output at any given time
- Safety — how easily batteries can short circuit, or toxic their internal chemistry might be
- Cycle life — how many times you can recharge your battery before it becomes unusable, and
- Cost
Each battery chemistry has its own particular mix of strengths within this matrix. The ideal battery, of course, would rank highly across all of them — and in that regard, lithium-ion comes very close. It’s incredibly energy dense, relatively safe, has a long cycle life, and is reasonably cheap to produce.
However, as we start placing more demands on our batteries, even lithium-ion batteries aren’t cutting it. As Martin Eberhard wrote, “One of the most difficult challenges in battery design is increasing energy density while also maximizing battery lifespan. Li-ion chemistries have achieved better combinations of these parameters than anything that has come before. Yet there is still a tradeoff between energy and life, even within the family of Li-ion.”
There’s still a long way to go to develop batteries which can cost-efficiently store the requisite amount of energy generated from renewables needed to fully electrify our infrastructure. For one thing, many renewable energy sources like solar panels and wind turbines generate energy at sporadic times in line with the weather. Without batteries that can store this generated energy for use at crucial moments, the energy that’s generated just goes to waste. According to Shirley Meng, a materials scientist at the University of Chicago studying batteries, “in the last century, about 60% of the energy we produced went to waste.”
Another outstanding issue is that even top-of-the-line lithium batteries are still not cost-competitive with gas-powered vehicle, furthermore, relying on just one battery chemistry, which depends on a dwindling supply of cobalt and lithium, is beginning to push prices up on batteries leading to a reversal of the the impressive price decline we’ve seen over the past few decades.
Confronting these challenges has become a top priority for entrepreneurs, scientists and governments around the world who are finally trying to get battery technology to catch up with the realities of the 21st century. To better understand the limits and future opportunities in batteries, let’s first take a look at the origins of this important technology.
The History of the Battery
In 1786, an Italian physician and biologist named Luigi Galvani was dissecting a frog. When he placed his scalpel to the frog’s leg, he was surprised to find the muscles came to life and the entire limb began to twitch. Earlier in his career, Galvani discovered that nerve impulses were electrochemical in nature. At the time, the scientific community was only in the very early innings of discovering the true nature of electricity. One important milestone was Benjamin Franklin’s realization that lightning and static electricity were of the same medium. The discovery made Franklin quite a celebrity in European scientific circles, even if his dangerous experiments with electricity made him a rather suspect character back home in America. However, getting to the core of what electricity was, and how it could be produced was a mystery still to be solved. Figuring out how to capture lightning in a bottle was one of the biggest scientific questions of the era. Thus, when Galvani’s scalpel induced what he knew to be an electrochemical impulse in the frog leg — a lightbulb went off.
Galvani was led to believe that electricity must be a substance inside of the frog that was triggered by the metal scalpel. He published these findings in a 1791 paper called “Commentary on the Effect of Electricity on Muscular Motion,” which was circulated amongst the scientists of the day, and eventually found itself in the hands of a professor of physics named Alessandro Volta.
Volta was unconvinced by Galvani’s explanation as he thought it highly improbable that the source of electricity was a latent fluid trapped inside of biological tissue. After re-creating Galvani’s experiment to the tee, Volta came to realize that the frog leg was not the true source of the electricity, but rather just a conduit for it. The real source of electricity came from the interaction of two disparate metals, the scalpel and a metal plate, being put into close proximity: the metal plate holding the frog was transferring its electrons through the frog leg to the metal in the scalpel. This exchange of electrons created a current, which interacted with the frog’s nerves, making the legs twitch.
Volta wanted to test this hypothesis, but this time, without using a frog as a conduit. He took two coins made of different metals and placed them on either side of his tongue. Instantly, he could feel a tinge of electric current pass between them. Next, he took plates of silver and zinc, separated by some cardboard soaked in salt water, and stacked them up one on top of the other. With one finger touching the plate of zinc at the bottom of the pile, and one finger touching that plate of silver at the top of the pile, Volta could feel a tiny electric shock. The ‘voltaic pile’ he had erected was producing an electrical current. It was the first battery ever made.
Volta’s invention predated knowledge of the atomic structure, so he merely chalked up the production of electricity to some chemical reaction created by the interaction of different metals. This wasn’t far off from the truth.
All atoms are inherently endowed with electric energy because all atoms are composed of charged particles, including protons and electrons. Today, we know that an atom’s structure determines how much electrochemical potential it has. Atoms that have a complete outer shell of electrons, like the noble gasses, are typically very stable and non-reactive. These elements have low electrochemical potential. Atoms with incomplete electron shells, on the other hand, want to react with surrounding atoms to reach a more stable structure and have more electrochemical potential. In Volta’s battery pile, zinc atoms were more reactive and willing to give up electrons, whereas the silver atoms were less reactive and willing to take on electrons. Placing a solution that could shuttle these electrons between the two metals, as Volta did by placing the salt-water soaked cardboard, was the key to creating an electric current inside the voltaic pile. With some experimentation, Volta also noted that by stacking a larger pile of zinc and silver plates, the strength of the current could be increased.
This structure is the basis of all batteries; a reactive material called an anode, willing to give up electrons on one end, a material willing to accept electrons called a cathode on the other end. The trick is to get the electrons from the anode to flow to the cathode, thus creating a current.
The issue is that the atoms in the anode and cathode are locked in solid structures, meaning they can’t move freely. To solve this, a solution called an electrolyte, with free-floating ions is placed as an intermediary between the two. The free-floating ions in the electrolyte act as a shuttle for electrons, picking them up at the anode end, and delivering them to the cathode end.
Volta’s battery was the first way we learned to convert chemical energy directly into electric energy. Before even knowing what electricity was, the battery was known as a reliable source of it. Before the existence of the energy grid or power stations, batteries were powering all kinds of early electric devices from the first electric motors to the telegraph.
The only issue was that early batteries were pretty inconvenient. They looked like jars with two metal rods stuck inside, the anode and the cathode, and were filled with a liquid, acidic electrolyte. By connecting a wire to the cathode end, you could power anything from a doorbell to a lightbulb, but when all the electrolytes in the solution were used up, the battery would stop producing a current. Then, you would have to service the battery by dumping out the acid, and refilling the jar with more electrolyte solution.
Being a ‘battery man’ was a real occupation at the time. Every major telegraph company, like Western Union, had one whose sole job it was to maintain and service the company’s massive gurgling, acidic batteries powering the telegraph network.
In his book “The Battery'', Henry Schlesinger writes about the debates that early engineers had regarding what kind of infrastructure might be required to electrify households. It was not entirely obvious at the time that each country would build out a massive electrical grid connecting every home to giant power stations. Many imagined that each home would need their own supply of batteries, delivered on a regular basis. In the same way that the milkman delivered milk in bottles to every home on a consistent schedule, it was conceivable that households would put out their empty battery jars every couple of days and get a fresh supply delivered. Another strange, though creative idea discussed in the book was installing a giant subterranean battery under the home whose electrolyte could be dispensed and replenished in the same way that rural homes dispense of the contents of their septic tanks.
Clearly the process of draining and refilling these batteries with corrosive, liquid substances was not opportune. The other problem with early battery designs is that they were not rechargeable. The electrolyte was producing irreversible chemical reactions with the anode and cathode material where eventually, no reaction would take place, no matter how many times you refilled the electrolyte. Chemists had a hunch that it might be possible to create a battery that created an electric current through a reversible chemical reaction, which would theoretically mean that, after depleted, you could apply a current to the cathode end to reverse the reaction, thus “recharging” the battery. The first such design was created by Gaston Planté in 1859. He had spent thirty years developing a rechargeable battery, and ultimately accomplished it using lead electrodes and an acidic electrolyte intermediary. When the battery was depleted, adding a current to the cathode end would, over time, recharge it, and allow it to function again.
Newfangled electric power spurred the consumer to accept all kinds of new electric gadgets into their home with great excitement. Still, the liquid, corrosive electrolytes powering the batteries were unwieldy and dangerous. No one wanted a box of acid sitting next to their telephones. In 1888, a “dry cell” was invented by merely mixing the electrolyte with a paste to create a kind of gel which sat between the anode and cathode parts. Initial versions of the dry cell were non-rechargeable, but they were so useful, could be made very compact and in a few decades were standardized and became common features in all kinds of consumer devices ranging from tooth brushes to children’s toys. Today’s Duracell batteries are still manufactured using the same dry cell model from the late 19th century.
The final evolution in battery design came from a yearning to complete the trifecta of rechargeable, high power, long-lasting batteries. Various types of battery chemistries were developed, like nickel-cadmium, which could supply enough juice to work a high power laptop, but were also toxic and would die out quickly if you recharged them before they were fully depleted.
The solution to all these problems and more was lithium. Lithium is the smallest atomic metal that exists. It has only two protons, making it incredibly light, and just one electron in its outer shell, making it incredibly reactive. Lithium’s natural reactivity made it an incredible chemical candidate in battery production — but its natural instability also made it volatile, and very prone to exploding or catching fire whenever pure lithium got into contact with other substances. Though the US was one of the first to begin studies on lithium batteries, it was ultimately the scientists at Sony and the Asahi Chemical Company which ultimately figured out a workable design around the 1990s. In their model, rather than using lithium as a pure anode, lithium-ion atoms would play the role of the electrolyte. They would pick up electrons from a porous, but ultimately stable anode end, and move across a semipermeable membrane over to the cathode end, generating a current. The remarkable part of this technology was that these reactions were reversible, which meant these batteries would be fully rechargeable!
Superior in nearly every way from the batteries of yore, lithium-ion batteries won the day and have been the reigning product in the battery market since the early 2000s.
The Trade-Offs Ahead
Onereason batteries are so exciting is that they are some of the most efficient ways of generating electrical energy. Unlike a traditional power plant, which turns chemical energy (lighting a combustible fuel) into thermal energy (boiling water to produce steam) into mechanical energy (turning a magnetic turbine) to finally achieve electrical energy, a battery converts chemical energy directly into electricity.
For comparison , coal power plants have an energy efficiency of around 30%, meaning they convert only 30% of the energy locked in the coal into electricity. The rest is lost through all the additional energy transitions required along the way. A fuel-powered vehicle is even less efficient than that. Only about 12-30% of the energy from the fuel you put in it is used to actually make its wheels turn. Since batteries are able to directly generate the electricity that can power a vehicle’s drive train, they’re a lot closer to a 100% energy conversion rate.
The crux of the matter is that while fuel offers an extremely inefficient energy conversion, it is incredibly energy dense. Fuel is so plentiful and has so much potential energy packed inside of it per liter that it doesn’t matter it’s only sub-30% efficient (at least for now). Batteries have the opposite profile; while they are extremely efficient at energy conversion, they have an energy density that is still 100 times less than that of gasoline. When factoring in that only 12-30% of the energy in gasoline actually goes into moving your vehicle, the comparison becomes closer, but batteries still lag behind.
Energy density is really just a matter of how many charged particles you can pack into a given volume. Lithium is already the smallest metal atom we have, and the most reactive at that. So to achieve even greater energy density from here on out will really require some clever solutions and imagination from scientists.
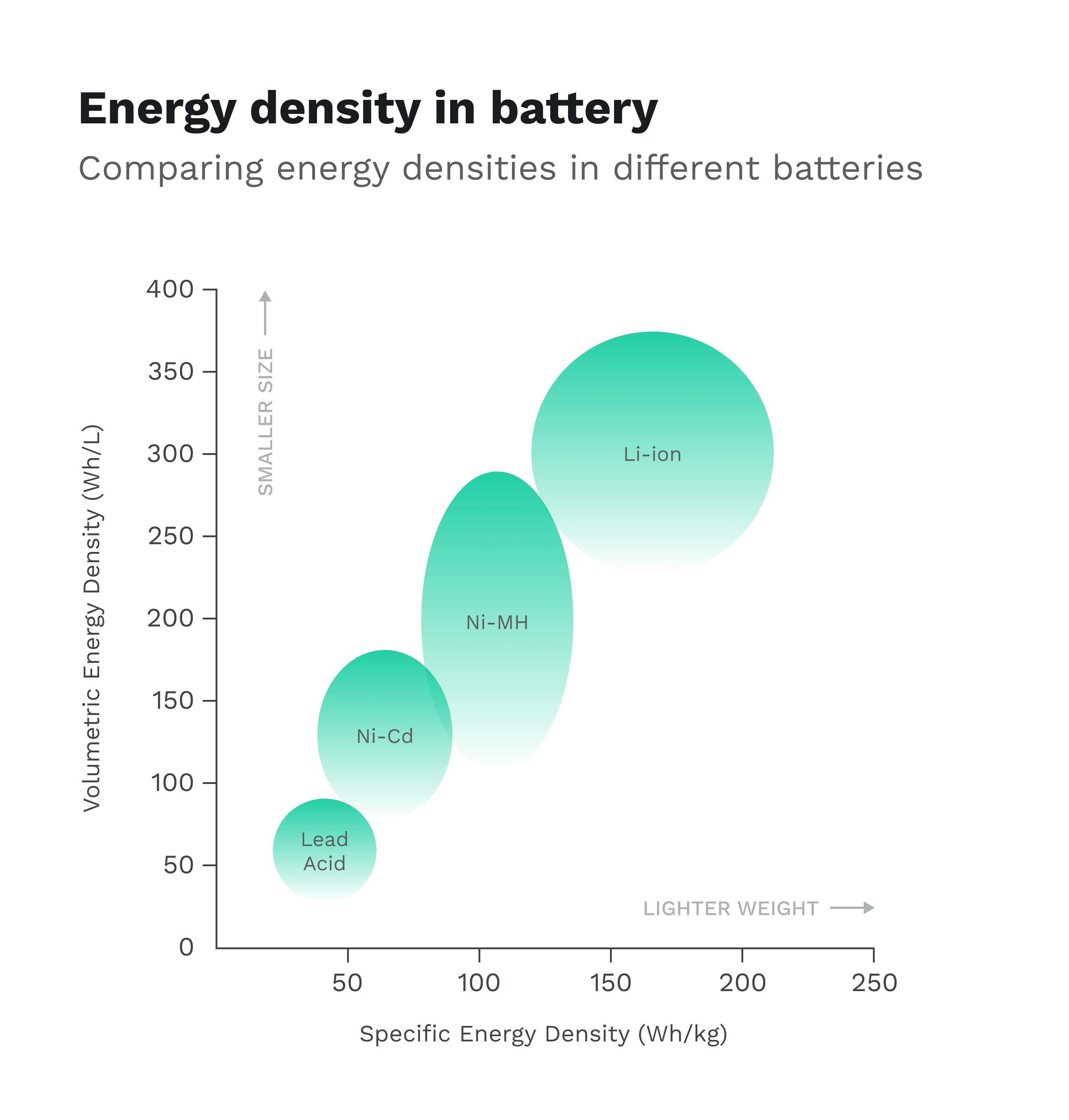
Source: Dragonfly Energy
For this reason, it might just be that the path forward will just look like a series of incremental gains. As Martin Eberhard already pointed out, building a better battery has always been a matter of choosing between different trade-offs. Do you value higher energy density or higher safety? More power output or a longer life cycle? For an excellent comparison of all the advantages and disadvantages of existing battery chemistries, take a look at the matrix compiled by Sarah Constantin.
Despite these challenges, there are still dozens of companies and researchers working on pushing out the frontier of battery capability. One option on the table is producing a battery with a lithium-metal anode. This would basically involve packing even more charged lithium particles into the battery to give it a higher energy density. Though this idea has been around for decades, making such a dense mesh of highly reactive lithium has always been looked upon as dangerous. Early iterations of these batteries have failed and short-circuited as a result, but companies like QuantumScape are now confident they’re on the verge of new advancements for the technology.
Other impressive teams, like Natron Energy or Enerpoly, which are primarily focused on creating battery alternatives for a world where we might become supply-constrained or priced out of using lithium-ion tech, are developing batteries which make use of sodium-ions — the second lightest atomic metal after lithium — or rechargeable zinc-ion batteries, respectively. These resources required to build these batteries are more equally distributed around the world.
Alongside the great research being embarked on today, researchers like Shirley Meng of the University of Chicago are still convinced that rather than waiting around for an ideal one-size-fits-all cell, we will need to begin operating in a world where certain battery chemistries, along with their trade-offs, will be best suited for some use cases over others.
The world is on an exciting path forward as it searches for ever more efficient and renewable energy sources to power its activities. Batteries will continue being not only an important puzzle to solve in the journey ahead, but also one whose breakthroughs will engender a host of new and exciting technological applications, just as the lithium-ion has already done for us in the modern day.
Disclosure: Nothing presented within this article is intended to constitute legal, business, investment or tax advice, and under no circumstances should any information provided herein be used or considered as an offer to sell or a solicitation of an offer to buy an interest in any investment fund managed by Contrary LLC (“Contrary”) nor does such information constitute an offer to provide investment advisory services. Information provided reflects Contrary’s views as of a time, whereby such views are subject to change at any point and Contrary shall not be obligated to provide notice of any change. Companies mentioned in this article may be a representative sample of portfolio companies in which Contrary has invested in which the author believes such companies fit the objective criteria stated in commentary, which do not reflect all investments made by Contrary. No assumptions should be made that investments listed above were or will be profitable. Due to various risks and uncertainties, actual events, results or the actual experience may differ materially from those reflected or contemplated in these statements. Nothing contained in this article may be relied upon as a guarantee or assurance as to the future success of any particular company. Past performance is not indicative of future results. A list of investments made by Contrary (excluding investments for which the issuer has not provided permission for Contrary to disclose publicly, Fund of Fund investments and investments in which total invested capital is no more than $50,000) is available at www.contrary.com/investments.
Certain information contained in here has been obtained from third-party sources, including from portfolio companies of funds managed by Contrary. While taken from sources believed to be reliable, Contrary has not independently verified such information and makes no representations about the enduring accuracy of the information or its appropriateness for a given situation. Charts and graphs provided within are for informational purposes solely and should not be relied upon when making any investment decision. Please see www.contrary.com/legal for additional important information.